Spironolactone Eyedrop Favors Restoration of Corneal Integrity after Wound Healing in the Rat
- PMID: 37895917
- PMCID: PMC10609951
- DOI: 10.3390/ph16101446
Spironolactone Eyedrop Favors Restoration of Corneal Integrity after Wound Healing in the Rat
Abstract
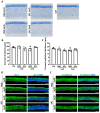
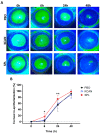
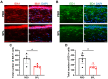
Abnormal corneal wound healing can compromise corneal transparency and lead to visual impairment. Mineralocorticoid receptor antagonists (MRA) are promising candidates to promote corneal remodeling with anti-inflammatory properties and lack gluococorticoids-associated side effects. In this preclinical study, a new polymer-free hydroxypropyl-gamma-cyclodextrin-based eyedrop containing 0.1% spironolactone (SPL), a potent but non-water-soluble MRA, was investigated for its ocular surface tolerance and efficacy in a rat model of corneal wound healing. SPL eyedrops were stable for up to 9 months at 4 °C. The formulation was well-tolerated since no morphological changes or inflammatory reactions were observed in the rat cornea after multiple daily instillations over 7 days. SPL eyedrops accelerated rat corneal wound healing, reduced corneal edema and inflammation, enhanced epithelial integrity, and improved nerve regeneration, suggesting restoration of corneal homeostasis, while potassium canrenoate, an active and soluble metabolite of SPL, had no effect. SPL eyedrops could benefit patients with impaired corneal wound healing, including that secondary to glucocorticoid therapy. Repurposing known drugs with known excipients will expedite translation to the clinic.
Keywords: cornea; corneal wound healing; drug development; eyedrop; ocular surface; spironolactone; topical administration.
Conflict of interest statement
J.-P.C. and X.C. are employees of Excelvision, Fareva, Annonay, France. Y.B. is an employee of Valdepharm, Fareva, Val-de-Reuil, France. P.R. is an employee of Fareva, France. D.R.-B., L.Z., E.G., A.T., J.-L.B., F.B.-C. and M.Z. declare no conflict of interest.
Figures






Similar articles
-
Topical Administration of Spironolactone-Loaded Nanomicelles Prevents Glucocorticoid-Induced Delayed Corneal Wound Healing in Rabbits.Mol Pharm. 2018 Mar 5;15(3):1192-1202. doi: 10.1021/acs.molpharmaceut.7b01028. Epub 2018 Feb 15. Mol Pharm. 2018. PMID: 29397733
-
Polymeric micelle mediated follicular delivery of spironolactone: Targeting the mineralocorticoid receptor to prevent glucocorticoid-induced activation and delayed cutaneous wound healing.Int J Pharm. 2021 Jul 15;604:120773. doi: 10.1016/j.ijpharm.2021.120773. Epub 2021 Jun 4. Int J Pharm. 2021. PMID: 34090990
-
Safety profile of topical VEGF neutralization at the cornea.Invest Ophthalmol Vis Sci. 2009 May;50(5):2095-102. doi: 10.1167/iovs.07-1129. Epub 2009 Jan 17. Invest Ophthalmol Vis Sci. 2009. PMID: 19151400
-
[The cornea: stasis and dynamics].Nippon Ganka Gakkai Zasshi. 2008 Mar;112(3):179-212; discussion 213. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18411711 Review. Japanese.
-
Targeting Ca2+-dependent pathways to promote corneal epithelial wound healing induced by CISD2 deficiency.Cell Signal. 2023 Sep;109:110755. doi: 10.1016/j.cellsig.2023.110755. Epub 2023 Jun 12. Cell Signal. 2023. PMID: 37315750 Review.
Cited by
-
Therapeutic Targets in the Management of Dry Eye Disease Associated with Sjögren's Syndrome: An Updated Review of Current Insights and Future Perspectives.J Clin Med. 2024 Mar 20;13(6):1777. doi: 10.3390/jcm13061777. J Clin Med. 2024. PMID: 38542002 Free PMC article. Review.
-
A Systematic Review of Spironolactone Nano-Formulations for Topical Treatment of Skin Hyperandrogenic Disorders and Chronic Wounds.Pharmaceutics. 2024 Dec 27;17(1):27. doi: 10.3390/pharmaceutics17010027. Pharmaceutics. 2024. PMID: 39861676 Free PMC article. Review.
-
Topical Ocular Drug Delivery: The Impact of Permeation Enhancers.Pharmaceutics. 2025 Mar 31;17(4):447. doi: 10.3390/pharmaceutics17040447. Pharmaceutics. 2025. PMID: 40284442 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

