Drug-Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly
- PMID: 37895930
- PMCID: PMC10610014
- DOI: 10.3390/ph16101457
Drug-Drug Interactions between Tamsulosin and Mirabegron in Healthy Individuals Do Not Affect Pharmacokinetics and Hemodynamic Parameters Significantly
Abstract
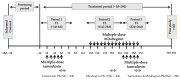
Overactive bladder (OAB) is characterized by urinary urgency and increased urinary frequency, substantially affecting quality of life. Tamsulosin and mirabegron combination therapy has been studied as a safe and effective treatment option for patients with OAB. This study evaluated the effects of combining these two drugs on their pharmacokinetics and safety profiles in healthy Korean males. In this open-label, fixed-sequence, three-period, drug-drug interaction phase 1 study, a total of 36 male participants were administered multiple doses of tamsulosin alone (0.2 mg once daily), mirabegron alone (50 mg once daily), or a combination of both drugs. The results showed that the combination of tamsulosin and mirabegron increased tamsulosin exposure in the plasma by approximately 40%. In contrast, the maximum plasma concentration of mirabegron was reduced by approximately 17% when administered with tamsulosin. No clinically significant changes in the safety profiles, vital signs, or clinical laboratory test results were observed in this study. In conclusion, there were no clinically relevant drug-drug interactions between tamsulosin and mirabegron in terms of pharmacokinetics, safety, and tolerability, suggesting that their combination could be a promising treatment option for patients with OAB.
Keywords: drug–drug interaction; mirabegron; pharmacokinetics; tamsulosin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Gormley E.A., Lightner D.J., Faraday M., Vasavada S.P., American Urological Association. Society of Urodynamics, Female Pelvic Medicine Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J. Urol. 2015;193:1572–1580. - PubMed
-
- Vrijens D., Drossaerts J., van Koeveringe G., Van Kerrebroeck P., van Os J., Leue C. Affective symptoms and the overactive bladder—A systematic review. J. Psychosom. Res. 2015;78:95–108. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources