Thiadiazolidinone (TDZD) Analogs Inhibit Aggregation-Mediated Pathology in Diverse Neurodegeneration Models, and Extend C. elegans Life- and Healthspan
- PMID: 37895969
- PMCID: PMC10610358
- DOI: 10.3390/ph16101498
Thiadiazolidinone (TDZD) Analogs Inhibit Aggregation-Mediated Pathology in Diverse Neurodegeneration Models, and Extend C. elegans Life- and Healthspan
Abstract
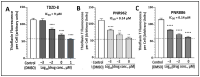
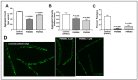
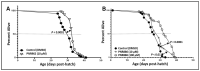
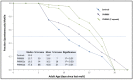
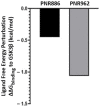
Chronic, low-grade inflammation has been implicated in aging and age-dependent conditions, including Alzheimer's disease, cardiomyopathy, and cancer. One of the age-associated processes underlying chronic inflammation is protein aggregation, which is implicated in neuroinflammation and a broad spectrum of neurodegenerative diseases such as Alzheimer's, Huntington's, and Parkinson's diseases. We screened a panel of bioactive thiadiazolidinones (TDZDs) from our in-house library for rescue of protein aggregation in human-cell and C. elegans models of neurodegeneration. Among the tested TDZD analogs, PNR886 and PNR962 were most effective, significantly reducing both the number and intensity of Alzheimer-like tau and amyloid aggregates in human cell-culture models of pathogenic aggregation. A C. elegans strain expressing human Aβ1-42 in muscle, leading to AD-like amyloidopathy, developed fewer and smaller aggregates after PNR886 or PNR962 treatment. Moreover, age-progressive paralysis was reduced 90% by PNR886 and 75% by PNR962, and "healthspan" (the median duration of spontaneous motility) was extended 29% and 62%, respectively. These TDZD analogs also extended wild-type C. elegans lifespan by 15-30% (p < 0.001), placing them among the most effective life-extension drugs. Because the lead drug in this family, TDZD-8, inhibits GSK3β, we used molecular-dynamic tools to assess whether these analogs may also target GSK3β. In silico modeling predicted that PNR886 or PNR962 would bind to the same allosteric pocket of inactive GSK3β as TDZD-8, employing the same pharmacophore but attaching with greater avidity. PNR886 and PNR962 are thus compelling candidate drugs for treatment of tau- and amyloid-associated neurodegenerative diseases such as AD, potentially also reducing all-cause mortality.
Keywords: Alzheimer’s disease; aggregation; glycogen synthase kinase 3β (GSK3β); molecular modeling; neurodegeneration; neurodegenerative disease; protein aggregation; proteostasis; thiadiazolidinones (TDZDs).
Conflict of interest statement
The authors declare no financial conflict of interest apart from intellectual property rights for drugs described herein. RJSR, PAC, SA, SK, SB, NRP, and others hold a patent on analogues PNR886 and PNR962. The University of Arkansas for Medical Sciences (UAMS) and the U.S. Government (Dept. of Veteran Affairs) jointly submitted patent claims on compounds PNR502, PNR886, and PNR962,
Figures



References
-
- Gandhi J., Antonelli A.C., Afridi A., Vatsia S., Joshi G., Romanov V., Murray I.V.J., Khan S.A. Protein Misfolding and Aggregation in Neurodegenerative Diseases: A Review of Pathogeneses, Novel Detection Strategies, and Potential Therapeutics. Rev. Neurosci. 2019;30:339–358. doi: 10.1515/revneuro-2016-0035. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

