Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP
- PMID: 37896128
- PMCID: PMC10609757
- DOI: 10.3390/pharmaceutics15102368
Evaluation of a Novel Dry Powder Surfactant Aerosol Delivery System for Use in Premature Infants Supported with Bubble CPAP
Abstract
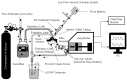
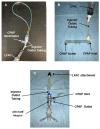
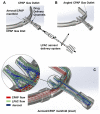
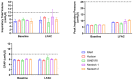
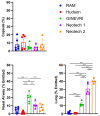
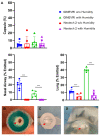
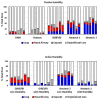
Aerosolized lung surfactant therapy during nasal continuous positive airway pressure (CPAP) support avoids intubation but is highly complex, with reported poor nebulizer efficiency and low pulmonary deposition. The study objective was to evaluate particle size, operational compatibility, and drug delivery efficiency with various nasal CPAP interfaces and gas humidity levels of a synthetic dry powder (DP) surfactant aerosol delivered by a low-flow aerosol chamber (LFAC) inhaler combined with bubble nasal CPAP (bCPAP). A particle impactor characterized DP surfactant aerosol particle size. Lung pressures and volumes were measured in a preterm infant nasal airway and lung model using LFAC flow injection into the bCPAP system with different nasal prongs. The LFAC was combined with bCPAP and a non-heated passover humidifier. DP surfactant mass deposition within the nasal airway and lung was quantified for different interfaces. Finally, surfactant aerosol therapy was investigated using select interfaces and bCPAP gas humidification by active heating. Surfactant aerosol particle size was 3.68 µm. Lung pressures and volumes were within an acceptable range for lung protection with LFAC actuation and bCPAP. Aerosol delivery of DP surfactant resulted in variable nasal airway (0-20%) and lung (0-40%) deposition. DP lung surfactant aerosols agglomerated in the prongs and nasal airways with significant reductions in lung delivery during active humidification of bCPAP gas. Our findings show high-efficiency delivery of small, synthetic DP surfactant particles without increasing the potential risk for lung injury during concurrent aerosol delivery and bCPAP with passive humidification. Specialized prongs adapted to minimize extrapulmonary aerosol losses and nasal deposition showed the greatest lung deposition. The use of heated, humidified bCPAP gases compromised drug delivery and safety. Safety and efficacy of DP aerosol delivery in preterm infants supported with bCPAP requires more research.
Keywords: aerosol delivery; bubble CPAP; dry powder lung surfactant; humidification; nasal prongs; nebulizer; particle size; preterm infant airway model; synthetic lung surfactant.
Conflict of interest statement
Robert DiBlasi has served as a consultant, received research funding, and has been on the speaker’s bureau for Draeger Medical, Vapotherm, and Medtronic. Frans Walther and The Lundquist Institute hold a patent on the B-YL SP-B peptide mimic (US 10,717,777).
Figures
Similar articles
-
Efficacy, dose-response, and aerosol delivery of dry powder synthetic lung surfactant treatment in surfactant-deficient rabbits and premature lambs.Respir Res. 2022 Apr 4;23(1):78. doi: 10.1186/s12931-022-02007-8. Respir Res. 2022. PMID: 35379243 Free PMC article.
-
In vitro evaluation of radio-labeled aerosol delivery via a variable-flow infant CPAP system.Respir Care. 2014 Mar;59(3):340-4. doi: 10.4187/respcare.01904. Epub 2013 Aug 6. Respir Care. 2014. PMID: 23920215
-
Aerosol delivery of dry powder synthetic lung surfactant to surfactant-deficient rabbits and preterm lambs on non-invasive respiratory support.Gates Open Res. 2019 Mar 14;3:6. doi: 10.12688/gatesopenres.12899.2. eCollection 2019. Gates Open Res. 2019. PMID: 31131369 Free PMC article.
-
Nasal high flow therapy for primary respiratory support in preterm infants.Cochrane Database Syst Rev. 2023 May 5;5(5):CD006405. doi: 10.1002/14651858.CD006405.pub4. Cochrane Database Syst Rev. 2023. PMID: 37144837 Free PMC article. Review.
-
Aerosol Delivery of Lung Surfactant and Nasal CPAP in the Treatment of Neonatal Respiratory Distress Syndrome.Front Pediatr. 2022 Jun 15;10:923010. doi: 10.3389/fped.2022.923010. eCollection 2022. Front Pediatr. 2022. PMID: 35783301 Free PMC article. Review.
Cited by
-
The Effect of Salbutamol and Budesonide Pediatric Doses on Dental Enamel and Packable and Flowable Composites: Microhardness, Surface Roughness and Color.Pharmaceutics. 2023 Oct 25;15(11):2527. doi: 10.3390/pharmaceutics15112527. Pharmaceutics. 2023. PMID: 38004507 Free PMC article.
-
Development of CPAP Overlay Interfaces for Efficient Administration of Aerosol Surfactant Therapy to Preterm Infants.AAPS PharmSciTech. 2025 Jan 16;26(1):34. doi: 10.1208/s12249-024-02987-4. AAPS PharmSciTech. 2025. PMID: 39821052
-
What is new in synthetic lung surfactant protein technology?Expert Rev Respir Med. 2024 Dec;18(12):913-917. doi: 10.1080/17476348.2024.2429669. Epub 2024 Nov 17. Expert Rev Respir Med. 2024. PMID: 39534910 No abstract available.
-
Development of a New Dry Powder Aerosol Synthetic Lung Surfactant Product for Neonatal Respiratory Distress Syndrome (RDS) - Part II: In vivo Efficacy Testing in a Rabbit Surfactant Washout Model.Pharm Res. 2024 Sep;41(9):1827-1842. doi: 10.1007/s11095-024-03754-7. Epub 2024 Sep 5. Pharm Res. 2024. PMID: 39237797 Free PMC article.
-
Moving on from clinical animal-derived surfactants to peptide-based synthetic pulmonary surfactant.Am J Physiol Lung Cell Mol Physiol. 2024 Dec 1;327(6):L883-L889. doi: 10.1152/ajplung.00186.2024. Epub 2024 Oct 15. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39404798 Free PMC article. Review.
References
-
- Wu A., Mukhtar-Yola M., Luch S., John S., Adhikari B.R., Bakker C., Slusher T., Bjorklund A., Winter J., Ezeaka C. Innovations and adaptations in neonatal and pediatric respiratory care for resource constrained settings. Front. Pediatr. 2022;10:954975. doi: 10.3389/fped.2022.954975. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

