Mechanistic Insight into the Early Stages of Toroidal Pore Formation by the Antimicrobial Peptide Smp24
- PMID: 37896158
- PMCID: PMC10610086
- DOI: 10.3390/pharmaceutics15102399
Mechanistic Insight into the Early Stages of Toroidal Pore Formation by the Antimicrobial Peptide Smp24
Abstract
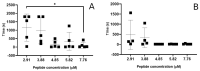
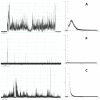
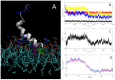
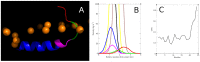
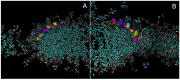
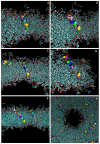
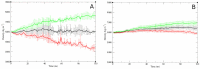
The antimicrobial peptide Smp24, originally derived from the venom of Scorpio maurus palmatus, is a promising candidate for further drug development. However, before doing so, greater insight into the mechanism of action is needed to construct a reliable structure-activity relationship. The aim of this study was to specifically investigate the critical early stages of peptide-induced membrane disruption. Single-channel current traces were obtained via planar patch-clamp electrophysiology, with multiple types of pore-forming events observed, unlike those expected from the traditional, more rigid mechanistic models. To better understand the molecular-level structures of the peptide-pore assemblies underlying these observed conductance events, molecular dynamics simulations were used to investigate the peptide structure and orientation both before and during pore formation. The transition of the peptides to transmembrane-like states within disordered toroidal pores occurred due to a peptide-induced bilayer-leaflet asymmetry, explaining why pore stabilization does not always follow pore nucleation in the experimental observations. To fully grasp the structure-activity relationship of antimicrobial peptides, a more nuanced view of the complex and dynamic mechanistic behaviour must be adopted.
Keywords: antimicrobial peptides; disordered toroidal pore; early-stage pore formation; mechanism of action; membrane pore; molecular dynamics simulations; patch-clamp electrophysiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
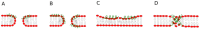
Similar articles
-
Phospholipid dependent mechanism of smp24, an α-helical antimicrobial peptide from scorpion venom.Biochim Biophys Acta. 2016 Nov;1858(11):2737-2744. doi: 10.1016/j.bbamem.2016.07.018. Epub 2016 Jul 30. Biochim Biophys Acta. 2016. PMID: 27480803
-
Scorpion Venom Antimicrobial Peptides Induce Siderophore Biosynthesis and Oxidative Stress Responses in Escherichia coli.mSphere. 2021 May 12;6(3):e00267-21. doi: 10.1128/mSphere.00267-21. mSphere. 2021. PMID: 33980680 Free PMC article.
-
Improving the Therapeutic Index of Smp24, a Venom-Derived Antimicrobial Peptide: Increased Activity against Gram-Negative Bacteria.Int J Mol Sci. 2022 Jul 20;23(14):7979. doi: 10.3390/ijms23147979. Int J Mol Sci. 2022. PMID: 35887325 Free PMC article.
-
Advances in Molecular Understanding of α-Helical Membrane-Active Peptides.Acc Chem Res. 2021 May 4;54(9):2196-2204. doi: 10.1021/acs.accounts.1c00047. Epub 2021 Apr 12. Acc Chem Res. 2021. PMID: 33844916 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Staphylococcus hominis as a source of antimicrobial peptides: identification of a new peptide with potential antimicrobial properties using in silico approach.Arch Microbiol. 2025 Apr 11;207(5):119. doi: 10.1007/s00203-025-04323-1. Arch Microbiol. 2025. PMID: 40214775
-
The Role of Flexibility in the Bioactivity of Short α-Helical Antimicrobial Peptides.Antibiotics (Basel). 2025 Apr 22;14(5):422. doi: 10.3390/antibiotics14050422. Antibiotics (Basel). 2025. PMID: 40426489 Free PMC article. Review.
-
Engineering of antimicrobial peptide Brevinin-1pl: arginine, lysine, and histidine substitutions enhance antimicrobial-anticancer efficacy with reduced cytotoxicity.Front Chem. 2025 May 19;13:1579097. doi: 10.3389/fchem.2025.1579097. eCollection 2025. Front Chem. 2025. PMID: 40458657 Free PMC article.
References
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kingdom; London, UK: 2016.
LinkOut - more resources
Full Text Sources

