Therapeutic Effects of Oral Application of Menthol and Extracts from Tormentil (Potentilla erecta), Raspberry Leaves (Rubus idaeus), and Loosestrife (Lythrum salicaria) during Acute Murine Campylobacteriosis
- PMID: 37896170
- PMCID: PMC10610364
- DOI: 10.3390/pharmaceutics15102410
Therapeutic Effects of Oral Application of Menthol and Extracts from Tormentil (Potentilla erecta), Raspberry Leaves (Rubus idaeus), and Loosestrife (Lythrum salicaria) during Acute Murine Campylobacteriosis
Abstract
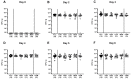
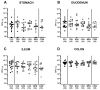
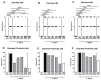
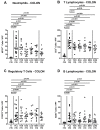
Human food-borne infections with the enteropathogen Campylobacter jejuni are becoming increasingly prevalent worldwide. Since antibiotics are usually not indicated in campylobacteriosis, alternative treatment regimens are important. We here investigated potential disease-alleviating effects of menthol and of extracts from tormentil, raspberry leaves, and loosestrife in acute murine campylobacteriosis. Therefore, C. jejuni-infected microbiota-depleted IL-10-/- mice were orally treated with the compounds alone or all in combination from day 2 until day 6 post-infection. Whereas neither treatment regimen affected gastrointestinal pathogen loads, the combination of compounds alleviated C. jejuni-induced diarrheal symptoms in diseased mice on day 6 post-infection. Furthermore, the therapeutic application of tormentil and menthol alone and the combination of the four compounds resulted in lower colonic T cell numbers in infected mice when compared to placebo counterparts. Notably, pro-inflammatory cytokines measured in mesenteric lymph nodes taken from C. jejuni-infected mice following tormentil, menthol, and combination treatment did not differ from basal concentrations. However, neither treatment regimen could dampen extra-intestinal immune responses, including systemic pro-inflammatory cytokine secretion on day 6 post-infection. In conclusion, the combination of menthol and of extracts from tormentil, raspberry leaves, and loosestrife constitutes an antibiotic-independent approach to alleviate campylobacteriosis symptoms.
Keywords: Campylobacter jejuni; acute campylobacteriosis; immune modulation; loosestrife (Lythrum salicaria); menthol; microbiota-depleted IL-10−/− mice; plant-derived compounds; raspberry leaves (Rubus idaeus); tormentil (Potentilla erecta).
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10-/- Mice.Biomolecules. 2024 Feb 29;14(3):290. doi: 10.3390/biom14030290. Biomolecules. 2024. PMID: 38540710 Free PMC article.
-
Oral curcumin ameliorates acute murine campylobacteriosis.Front Immunol. 2024 May 24;15:1363457. doi: 10.3389/fimmu.2024.1363457. eCollection 2024. Front Immunol. 2024. PMID: 38855111 Free PMC article.
-
Combination of organic acids benzoate, butyrate, caprylate, and sorbate provides a novel antibiotics-independent treatment option in the combat of acute campylobacteriosis.Front Microbiol. 2023 Mar 15;14:1128500. doi: 10.3389/fmicb.2023.1128500. eCollection 2023. Front Microbiol. 2023. PMID: 37007531 Free PMC article.
-
Human Campylobacteriosis-A Serious Infectious Threat in a One Health Perspective.Curr Top Microbiol Immunol. 2021;431:1-23. doi: 10.1007/978-3-030-65481-8_1. Curr Top Microbiol Immunol. 2021. PMID: 33620646 Review.
-
Raspberry Leaves and Extracts-Molecular Mechanism of Action and Its Effectiveness on Human Cervical Ripening and the Induction of Labor.Nutrients. 2023 Jul 19;15(14):3206. doi: 10.3390/nu15143206. Nutrients. 2023. PMID: 37513625 Free PMC article. Review.
Cited by
-
Therapeutic and protective approaches to combat Campylobacter jejuni infections.Front Pharmacol. 2025 May 6;16:1572616. doi: 10.3389/fphar.2025.1572616. eCollection 2025. Front Pharmacol. 2025. PMID: 40395728 Free PMC article. Review.
-
Menthol Pretreatment Alleviates Campylobacter jejuni-Induced Enterocolitis in Human Gut Microbiota-Associated IL-10-/- Mice.Biomolecules. 2024 Feb 29;14(3):290. doi: 10.3390/biom14030290. Biomolecules. 2024. PMID: 38540710 Free PMC article.
-
Traditionally Used Medicinal Plants of Armenia.Plants (Basel). 2024 Dec 4;13(23):3411. doi: 10.3390/plants13233411. Plants (Basel). 2024. PMID: 39683204 Free PMC article. Review.
References
-
- Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., Abbasi-Kangevari M., Abbastabar H., Abd-Allah F., Abdelalim A., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. - DOI - PMC - PubMed
-
- Berthenet E., Thépault A., Chemaly M., Rivoal K., Ducournau A., Buissonnière A., Bénéjat L., Bessède E., Mégraud F., Sheppard S.K., et al. Source attribution of Campylobacter jejuni shows variable importance of chicken and ruminants reservoirs in non-invasive and invasive French clinical isolates. Sci. Rep. 2019;9:8098. doi: 10.1038/s41598-019-44454-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

