Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas
- PMID: 37896172
- PMCID: PMC10610306
- DOI: 10.3390/pharmaceutics15102412
Lipid-Coated Polymeric Nanoparticles for the Photodynamic Therapy of Head and Neck Squamous Cell Carcinomas
Abstract
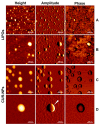
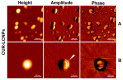
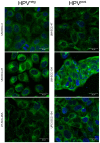
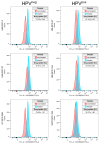
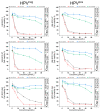
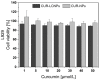
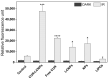
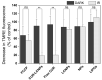
Next to alcohol and tobacco abuse, infection with human papillomaviruses (HPVs) is a major risk factor for developing head and neck squamous cell carcinomas (HNSCCs), leading to 350,000 casualties worldwide each year. Limited therapy options and drug resistance raise the urge for alternative methods such as photodynamic therapy (PDT), a minimally invasive procedure used to treat HNSCC and other cancers. We prepared lipid-coated polymeric nanoparticles encapsulating curcumin as the photosensitizer (CUR-LCNPs). The prepared CUR-LCNPs were in the nanometer range (153.37 ± 1.58 nm) and showed an encapsulation efficiency of 92.69 ± 0.03%. Proper lipid coating was visualized using atomic force microscopy (AFM). The CUR-LCNPs were tested in three HPVpos and three HPVneg HNSCC lines regarding their uptake capabilities and in vitro cell killing capacity, revealing a variable but highly significant tumor cell inhibiting effect in all tested HNSCC cell lines. No significant differences were detected between the HPVpos and HPVneg HNSCC groups (mean IC50: (9.34 ± 4.73 µmol/L vs. 6.88 ± 1.03 µmol/L), suggesting CUR-LCNPs/PDT to be a promising therapeutic option for HNSCC patients independent of their HPV status.
Keywords: HNSCC; LED; PDT; PLGA; cancer; curcumin; human papillomavirus; liposomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma.Cancer. 2017 May 15;123(10):1778-1790. doi: 10.1002/cncr.30570. Epub 2017 Mar 13. Cancer. 2017. PMID: 28295222 Free PMC article.
-
Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line.Photodiagnosis Photodyn Ther. 2018 Sep;23:190-201. doi: 10.1016/j.pdpdt.2018.06.026. Epub 2018 Jun 30. Photodiagnosis Photodyn Ther. 2018. PMID: 29969678
-
Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers.J Natl Cancer Inst. 2008 Mar 19;100(6):407-20. doi: 10.1093/jnci/djn025. Epub 2008 Mar 11. J Natl Cancer Inst. 2008. PMID: 18334711
-
A Review of HPV-Related Head and Neck Cancer.J Clin Med. 2018 Aug 27;7(9):241. doi: 10.3390/jcm7090241. J Clin Med. 2018. PMID: 30150513 Free PMC article. Review.
-
Human Papillomavirus Infection in Head and Neck Squamous Cell Carcinomas: Transcriptional Triggers and Changed Disease Patterns.Front Cell Infect Microbiol. 2020 Dec 2;10:537650. doi: 10.3389/fcimb.2020.537650. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33344262 Free PMC article. Review.
Cited by
-
The Efficacy of Curcumin-Mediated Photodynamic Therapy in the Treatment of Oral Squamous Cell Carcinoma: A Systematic Review of In Vitro Studies.Life (Basel). 2025 Jun 7;15(6):924. doi: 10.3390/life15060924. Life (Basel). 2025. PMID: 40566575 Free PMC article. Review.
-
Photodynamic Therapy for Oral Squamous Cell Carcinoma: Current Status, Challenges, and Prospects.Int J Nanomedicine. 2024 Oct 22;19:10699-10710. doi: 10.2147/IJN.S481901. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39464676 Free PMC article. Review.
-
Exploring the therapeutic potential of lipid-based nanoparticles in the management of oral squamous cell carcinoma.Explor Target Antitumor Ther. 2024;5(6):1223-1246. doi: 10.37349/etat.2024.00272. Epub 2024 Sep 29. Explor Target Antitumor Ther. 2024. PMID: 39465011 Free PMC article. Review.
-
Versatile Porphyrin Arrangements for Photodynamic Therapy-A Review.Nanomaterials (Basel). 2024 Nov 22;14(23):1879. doi: 10.3390/nano14231879. Nanomaterials (Basel). 2024. PMID: 39683268 Free PMC article. Review.
-
Harnessing curcumin and nanotechnology for enhanced treatment of breast cancer bone metastasis.Discov Nano. 2024 Nov 11;19(1):177. doi: 10.1186/s11671-024-04126-1. Discov Nano. 2024. PMID: 39527354 Free PMC article. Review.
References
-
- Haring C.T., Bhambhani C., Brummel C., Jewell B., Bellile E., Heft Neal M.E., Sandford E., Spengler R.M., Bhangale A., Spector M.E., et al. Human papilloma virus circulating tumor DNA assay predicts treatment response in recurrent/metastatic head and neck squamous cell carcinoma. Oncotarget. 2021;12:1214–1229. doi: 10.18632/oncotarget.27992. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

