Saquinavir-Piperine Eutectic Mixture: Preparation, Characterization, and Dissolution Profile
- PMID: 37896206
- PMCID: PMC10609941
- DOI: 10.3390/pharmaceutics15102446
Saquinavir-Piperine Eutectic Mixture: Preparation, Characterization, and Dissolution Profile
Abstract
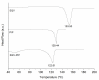
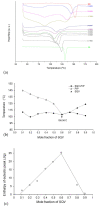
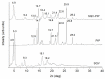
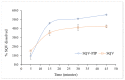
The dissolution rate of the anti-HIV drug saquinavir base (SQV), a poorly water-soluble and extremely low absolute bioavailability drug, was improved through a eutectic mixture formation approach. A screening based on a liquid-assisted grinding technique was performed using a 1:1 molar ratio of the drug and the coformers sodium saccharinate, theobromine, nicotinic acid, nicotinamide, vanillin, vanillic acid, and piperine (PIP), followed by differential scanning calorimetry (DSC). Given that SQV-PIP was the only resulting eutectic system from the screening, both the binary phase and the Tammann diagrams were adapted to this system using DSC data of mixtures prepared from 0.1 to 1.0 molar ratios in order to determine the exact eutectic composition. The SQV-PIP system formed a eutectic at a composition of 0.6 and 0.40, respectively. Then, a solid-state characterization through DSC, powder X-ray diffraction (PXRD), including small-angle X-ray scattering (SAXS) measurements to explore the small-angle region in detail, Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and a powder dissolution test were performed. The conventional PXRD analyses suggested that the eutectic mixture did not exhibit structural changes; however, the small-angle region explored through the SAXS instrument revealed a change in the crystal structure of one of their components. FT-IR spectra showed no molecular interaction in the solid state. Finally, the dissolution profile of SQV in the eutectic mixture was different from the dissolution of pure SQV. After 45 min, approximately 55% of the drug in the eutectic mixture was dissolved, while, for pure SQV, 42% dissolved within this time. Hence, this study concludes that the dissolution rate of SQV can be effectively improved through the approach of using PIP as a coformer.
Keywords: dissolution enhancement; eutectic mixtures; piperine; powder diffraction; saquinavir; small-angle X-ray scattering; solid-state characterization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Evaluation of Piperine as Natural Coformer for Eutectics Preparation of Drugs Used in the Treatment of Cardiovascular Diseases.AAPS PharmSciTech. 2022 Apr 26;23(5):127. doi: 10.1208/s12249-022-02270-4. AAPS PharmSciTech. 2022. PMID: 35474407
-
Preparation and characterization of glimepiride eutectic mixture with l-arginine for improvement of dissolution rate.Int J Pharm. 2020 May 15;581:119288. doi: 10.1016/j.ijpharm.2020.119288. Epub 2020 Mar 31. Int J Pharm. 2020. PMID: 32243966
-
Drug Solubility Enhancement through the Preparation of Multicomponent Organic Materials: Eutectics of Lovastatin with Carboxylic Acids.Pharmaceutics. 2019 Mar 9;11(3):112. doi: 10.3390/pharmaceutics11030112. Pharmaceutics. 2019. PMID: 30857331 Free PMC article.
-
Thermodynamic and Structural Characterization of a Mechanochemically Synthesized Pyrazinamide-Acetylsalicylic-Acid Eutectic Mixture.Pharmaceuticals (Basel). 2025 Feb 5;18(2):211. doi: 10.3390/ph18020211. Pharmaceuticals (Basel). 2025. PMID: 40006026 Free PMC article.
-
Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs.Int J Pharm. 2020 Oct 15;588:119741. doi: 10.1016/j.ijpharm.2020.119741. Epub 2020 Aug 9. Int J Pharm. 2020. PMID: 32783978 Review.
Cited by
-
Development of Sinomenine Hydrochloride Sustained-release Pellet With Multiple Release Characteristics.AAPS PharmSciTech. 2024 Sep 25;25(7):224. doi: 10.1208/s12249-024-02949-w. AAPS PharmSciTech. 2024. PMID: 39322795
References
-
- Bansal K., Pant P., Rao P.R.T., Padhee K., Sathapathy A., Kochhar P.S. Micronization and dissolution enhancement of norethindrone. Int. J. Res. Pharm. Chem. 2011;1:315–319.
Grants and funding
LinkOut - more resources
Full Text Sources

