Liposomes for Cancer Theranostics
- PMID: 37896208
- PMCID: PMC10610083
- DOI: 10.3390/pharmaceutics15102448
Liposomes for Cancer Theranostics
Abstract
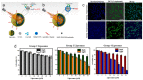
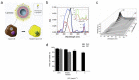
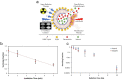
Cancer is one of the most well-studied diseases and there have been significant advancements over the last few decades in understanding its molecular and cellular mechanisms. Although the current treatments (e.g., chemotherapy, radiotherapy, gene therapy and immunotherapy) have provided complete cancer remission for many patients, cancer still remains one of the most common causes of death in the world. The main reasons for the poor response rates for different cancers include the lack of drug specificity, drug resistance and toxic side effects (i.e., in healthy tissues). For addressing the limitations of conventional cancer treatments, nanotechnology has shown to be an important field for constructing different nanoparticles for destroying cancer cells. Due to their size (i.e., less than 1 μm), nanoparticles can deliver significant amounts of cancer drugs to tumors and are able to carry moieties (e.g., folate, peptides) for targeting specific types of cancer cells (i.e., through receptor-mediated endocytosis). Liposomes, composed of phospholipids and an interior aqueous core, can be used as specialized delivery vehicles as they can load different types of cancer therapy agents (e.g., drugs, photosensitizers, genetic material). In addition, the ability to load imaging agents (e.g., fluorophores, radioisotopes, MRI contrast media) enable these nanoparticles to be used for monitoring the progress of treatment. This review examines a wide variety of different liposomes for cancer theranostics, with the different available treatments (e.g., photothermal, photodynamic) and imaging modalities discussed for different cancers.
Keywords: image-guided therapy; imaging; nanoparticles; theranostics; therapy.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Sharmiladevi P., Girigoswami K., Haribabu V., Girigoswami A. Nano-enabled theranostics for cancer. Mater. Adv. 2021;2:2876–2891. doi: 10.1039/D1MA00069A. - DOI
Publication types
LinkOut - more resources
Full Text Sources

