Clinical Pharmacology of Vinpocetine: Properties Revisited and Introduction of a Population Pharmacokinetic Model for Its Metabolite, Apovincaminic Acid (AVA)
- PMID: 37896263
- PMCID: PMC10610279
- DOI: 10.3390/pharmaceutics15102502
Clinical Pharmacology of Vinpocetine: Properties Revisited and Introduction of a Population Pharmacokinetic Model for Its Metabolite, Apovincaminic Acid (AVA)
Abstract
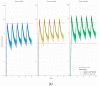
This paper examines the use of vinpocetine in the context of clinical pharmacology. The main and active metabolite of vinpocetine is apovincaminic acid (AVA). Due to the scarce information in the literature on AVA pharmacokinetics, we propose a population pharmacokinetic (PopPK) model for AVA based on a study in healthy volunteers with three different formulations of vinpocetine. The suggested PopPK model (and simulations) could be helpful in ensuring the more effective and safer use of the vinpocetine in the future given the increasing range of suggested indications for its use.
Keywords: apovincaminic acid; pharmacology; population pharmacokinetic model; simulation; vinpocetine.
Conflict of interest statement
The authors Z.P., P.P. and J.G.M. declare no conflict of interest. A.F. is an employee of Tecnimede, Portugal, EU. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Alkaloid Chemistry . In: Alkaloids-Secrets of Life. Aniszewski T., editor. Elsevier; Amsterdam, The Netherlands: 2007. pp. 61–139.
-
- Willson C. Elsevier Reference Module in Biomedical Sciences. Elsevier; Amsterdam, The Netherlands: 2015. Vinpocetine; pp. 1–6.
-
- NIH-National Center for Advancing Translational Sciences Apovincamine. [(accessed on 15 September 2023)]; Available online: https://drugs.ncats.io/drug/504R182ZX7.
LinkOut - more resources
Full Text Sources

