Computational Amendment of Parenteral In Situ Forming Particulates' Characteristics: Design of Experiment and PBPK Physiological Modeling
- PMID: 37896273
- PMCID: PMC10609842
- DOI: 10.3390/pharmaceutics15102513
Computational Amendment of Parenteral In Situ Forming Particulates' Characteristics: Design of Experiment and PBPK Physiological Modeling
Abstract
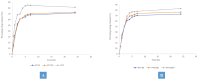
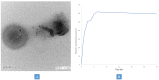
Lipid and/or polymer-based drug conjugates can potentially minimize side effects by increasing drug accumulation at target sites and thus augment patient compliance. Formulation factors can present a potent influence on the characteristics of the obtained systems. The selection of an appropriate solvent with satisfactory rheological properties, miscibility, and biocompatibility is essential to optimize drug release. This work presents a computational study of the effect of the basic formulation factors on the characteristics of the obtained in situ-forming particulates (IFPs) encapsulating a model drug using a 21.31 full factorial experimental design. The emulsion method was employed for the preparation of lipid and/or polymer-based IFPs. The IFP release profiles and parameters were computed. Additionally, a desirability study was carried out to choose the optimum formulation for further morphological examination, rheological study, and PBPK physiological modeling. Results revealed that the type of particulate forming agent (lipid/polymer) and the incorporation of structure additives like Brij 52 and Eudragit RL can effectively augment the release profile as well as the burst of the drug. The optimized formulation exhibited a pseudoplastic rheological behavior and yielded uniformly spherical-shaped dense particulates with a PS of 573.92 ± 23.5 nm upon injection. Physiological modeling simulation revealed the pioneer pharmacokinetic properties of the optimized formulation compared to the observed data. These results assure the importance of controlling the formulation factors during drug development, the potentiality of the optimized IFPs for the intramuscular delivery of piroxicam, and the reliability of PBPK physiological modeling in predicting the biological performance of new formulations with effective cost management.
Keywords: PBPK; PDLG; cholesterol; design of experiment; in situ forming nanoparticles; lipid; parenteral; polymer; targeted drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
SeDeM tool-driven full factorial design for osmotic drug delivery of tramadol HCl: Formulation development, physicochemical evaluation, and in-silico PBPK modeling for predictive pharmacokinetic evaluation using GastroPlus™.Front Pharmacol. 2022 Oct 7;13:974715. doi: 10.3389/fphar.2022.974715. eCollection 2022. Front Pharmacol. 2022. PMID: 36278217 Free PMC article.
-
Non-ionic Surfactant Based In Situ Forming Vesicles as Controlled Parenteral Delivery Systems.AAPS PharmSciTech. 2018 Apr;19(3):1001-1010. doi: 10.1208/s12249-017-0897-8. Epub 2017 Nov 6. AAPS PharmSciTech. 2018. PMID: 29110291
-
An integrated computational methodology with data-driven machine learning, molecular modeling and PBPK modeling to accelerate solid dispersion formulation design.Eur J Pharm Biopharm. 2021 Jan;158:336-346. doi: 10.1016/j.ejpb.2020.12.001. Epub 2020 Dec 7. Eur J Pharm Biopharm. 2021. PMID: 33301864
-
Progressive tools and critical strategies for development of best fit PBPK model aiming better in vitro-in vivo correlation.Int J Pharm. 2023 Aug 25;643:123267. doi: 10.1016/j.ijpharm.2023.123267. Epub 2023 Jul 22. Int J Pharm. 2023. PMID: 37488057 Review.
-
An overview of PLGA in-situ forming implants based on solvent exchange technique: effect of formulation components and characterization.Pharm Dev Technol. 2021 Sep;26(7):709-728. doi: 10.1080/10837450.2021.1944207. Epub 2021 Jun 27. Pharm Dev Technol. 2021. PMID: 34176433 Review.
References
-
- Trenfield S.J., Basit A.W. Modified Drug Release: Current Strategies and Novel Technologies for Oral Drug Delivery. In: Martins J.P., Santos H.A., editors. Nanotechnology for Oral Drug Delivery. Academic Press; Cambridge, MA, USA: 2020. pp. 177–197. Chapter 6.
Grants and funding
LinkOut - more resources
Full Text Sources

