Towards Effective Antiviral Oral Therapy: Development of a Novel Self-Double Emulsifying Drug Delivery System for Improved Zanamivir Intestinal Permeability
- PMID: 37896277
- PMCID: PMC10610354
- DOI: 10.3390/pharmaceutics15102518
Towards Effective Antiviral Oral Therapy: Development of a Novel Self-Double Emulsifying Drug Delivery System for Improved Zanamivir Intestinal Permeability
Abstract
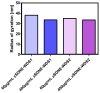
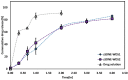
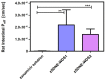
Self-double emulsifying drug delivery systems have the potential to enhance the intestinal permeability of drugs classified under the Biopharmaceutics Classification System (BCS) class III. One such example is the antiviral agent zanamivir, exhibiting suboptimal oral absorption (with a bioavailability range of 1-5%). To address this challenge, we have developed an innovative oral formulation for zanamivir: a self-double nanoemulsifying Winsor delivery system (SDNE-WDS) consisting of the microemulsion, which subsequently yields final double nanoemulsion (W1/O/W2) upon interaction with water. Two distinct formulations were prepared: SDNE-WDS1, classified as a W/O microemulsion, and SDNE-WDS2, discovered to be a bicontinuous microemulsion. The inner microemulsions displayed a consistent radius of gyration, with an average size of 35.1 ± 2.1 nm. Following self-emulsification, the resultant zanamivir-loaded nanoemulsion droplets for zSDNE-WDS1 and zSDNE-WDS2 measured 542.1 ± 36.1 and 174.4 ± 3.4 nm, respectively. Both types of emulsions demonstrated the ability to enhance the transport of zanamivir across a parallel artificial membrane. Additionally, in situ rat intestinal perfusion studies involving drug-loaded SDNE-WDSs revealed a significantly increased permeability of zanamivir through the small intestinal wall. Notably, both SDNE-WDS formulations exhibited effective permeability (Peff) values that were 3.5-5.5-fold higher than those of the low/high permeability boundary marker metoprolol. This research emphasizes the success of SDNE-WDSs in overcoming intestinal permeability barriers and enabling the effective oral administration of zanamivir. These findings hold promise for advancing the development of efficacious oral administration of BCS class III drugs.
Keywords: bioavailability; intestinal permeability; microemulsion; nanoemulsion; oral drug absorption; self-double emulsifying drug delivery system; zanamivir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Enabling oral delivery of antiviral drugs: Double emulsion carriers to improve the intestinal absorption of zanamivir.Int J Pharm. 2022 Dec 15;629:122392. doi: 10.1016/j.ijpharm.2022.122392. Epub 2022 Nov 13. Int J Pharm. 2022. PMID: 36379395
-
Enhancing Oral Bioavailability of Domperidone Maleate: Formulation, In vitro Permeability Evaluation In-caco-2 Cell Monolayers and In situ Rat Intestinal Permeability Studies.Curr Drug Deliv. 2024;21(7):1010-1023. doi: 10.2174/1567201820666230214091509. Curr Drug Deliv. 2024. PMID: 36786136 Free PMC article.
-
Enabling the intestinal absorption of highly polar antiviral agents: ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir.Mol Pharm. 2010 Aug 2;7(4):1223-34. doi: 10.1021/mp100050d. Mol Pharm. 2010. PMID: 20536260 Free PMC article.
-
Celecoxib Oral Solution and the Benefits of Self-Microemulsifying Drug Delivery Systems (SMEDDS) Technology: A Narrative Review.Pain Ther. 2023 Oct;12(5):1109-1119. doi: 10.1007/s40122-023-00529-7. Epub 2023 Jun 17. Pain Ther. 2023. PMID: 37329440 Free PMC article. Review.
-
Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects.Pharm Res. 1995 Nov;12(11):1561-72. doi: 10.1023/a:1016268311867. Pharm Res. 1995. PMID: 8592652 Review.
Cited by
-
Self-Emulsifying Drug Delivery Systems (SEDDS): Transition from Liquid to Solid-A Comprehensive Review of Formulation, Characterization, Applications, and Future Trends.Pharmaceutics. 2025 Jan 5;17(1):63. doi: 10.3390/pharmaceutics17010063. Pharmaceutics. 2025. PMID: 39861711 Free PMC article. Review.
References
-
- Center for Disease Control and Prevention. [(accessed on 15 September 2023)]; Available online: https://www.cdc.gov/flu/weekly/index.htm.
LinkOut - more resources
Full Text Sources

