Atomic Force Microscopy of Hydrolysed Polyacrylamide Adsorption onto Calcium Carbonate
- PMID: 37896286
- PMCID: PMC10609783
- DOI: 10.3390/polym15204037
Atomic Force Microscopy of Hydrolysed Polyacrylamide Adsorption onto Calcium Carbonate
Abstract
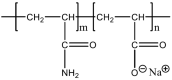
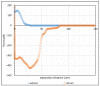
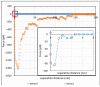
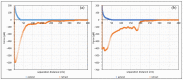
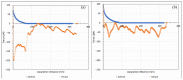
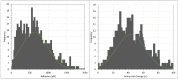
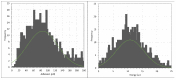
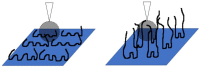
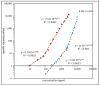
In this work, the interaction of hydrolysed polyacrylamide (HPAM) of two molecular weights (F3330, 11-13 MDa; F3530, 15-17 MDa) with calcium carbonate (CaCO3) was studied via atomic force microscopy (AFM). In the absence of polymers at 1.7 mM and 1 M NaCl, good agreement with DLVO theory was observed. At 1.7 mM NaCl, repulsive interaction during approach at approximately 20 nm and attractive adhesion of approximately 400 pN during retraction was measured, whilst, at 1 M NaCl, no repulsion during approach was found. Still, a significantly larger adhesion of approximately 1400 pN during retraction was observed. In the presence of polymers, results indicated that F3330 displayed higher average adhesion (450-625 pN) and interaction energy (43-145 aJ) with CaCO3 than F3530's average adhesion (85-88 pN) and interaction energy (8.4-11 aJ). On the other hand, F3530 exerted a longer steric repulsion distance (70-100 nm) than F3330 (30-70 nm). This was likely due to the lower molecular weight. F3330 adopted a flatter configuration on the calcite surface, creating more anchor points with the surface in the form of train segments. The adhesion and interaction energy of both HPAM with CaCO3 can be decreased by increasing the salt concentration. At 3% NaCl, the average adhesion and interaction energy of F3330 was 72-120 pN and 5.6-17 aJ, respectively, while the average adhesion and interaction energy of F3530 was 11.4-48 pN and 0.3-2.98 aJ, respectively. The reduction of adhesion and interaction energy was likely due to the screening of the COO- charged group of HPAM by salt cations, leading to a reduction of electrostatic attraction between the negatively charged HPAM and the positively charged CaCO3.
Keywords: atomic force microscopy (AFM); calcium carbonate; force spectroscopy; hydrolysed polyacrylamide; molecular weight.
Conflict of interest statement
Author Maung Maung Myo Thant was employed by the company PETRONAS Research Sdn. Bhd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Petroliam Nasional Berhad (PETRONAS). The funder had the following involvement with the study: Conceptualization.
Figures





References
-
- Wang S., Li G., Li Y., Guo J., Zhou S., Yong S., Pan B., Bai B. Adsorption of new hydrophobic polyacrylamide on the calcite surface. J. Appl. Polym. Sci. 2017;134:45314–45321. doi: 10.1002/app.45314. - DOI
-
- Mahmood A., Vissapragada B., Alghamdi A.H., Allen D., Herron M., Carnegie A., Dutta D., Olesen J.-R., Chourasiya R.D., Logan D., et al. A Snapshot of Carbonate Reservoir Evaluation. Oilfield Rev. 2000;12:20–41.
-
- Talaghat M.R., Esmaeilzadeh F., Mowla D. Sand production control by chemical consolidation. J. Pet. Sci. Eng. 2009;67:34–40. doi: 10.1016/j.petrol.2009.02.005. - DOI
-
- Nouri A., Vaziri H., Belhaj H., Islam R. Effect of Volumetric Failure on Sand Production in Oil-Wellbores; Proceedings of the SPE—Asia Pacific Oil and Gas Conference and Exhibition; Jakarta, Indonesia. 9–11 September 2003; pp. 86–93.
-
- Mowar S., Zaman M., Stearns D.W., Roegiers J.C. Micro-mechanisms of pore collapse in limestone. J. Pet. Sci. Eng. 1996;15:221–235. doi: 10.1016/0920-4105(95)00065-8. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

