Manipulating Molecular Self-Assembly Process at the Solid-Liquid Interface Probed by Scanning Tunneling Microscopy
- PMID: 37896420
- PMCID: PMC10610993
- DOI: 10.3390/polym15204176
Manipulating Molecular Self-Assembly Process at the Solid-Liquid Interface Probed by Scanning Tunneling Microscopy
Abstract
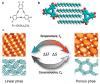
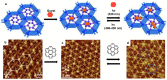
The phenomenon of ordered self-assembly on solid substrates is a topic of interest in both fundamental surface science research and its applications in nanotechnology. The regulation and control of two-dimensional (2D) self-assembled supra-molecular structures on surfaces have been realized through applying external stimuli. By utilizing scanning tunneling microscopy (STM), researchers can investigate the detailed phase transition process of self-assembled monolayers (SAMs), providing insight into the interplay between intermolecular weak interactions and substrate-molecule interactions, which govern the formation of molecular self-assembly. This review will discuss the structural transition of self-assembly probed by STM in response to external stimuli and provide state-of-the-art methods such as tip-induced confinement for the alignment of SAM domains and selective chirality. Finally, we discuss the challenges and opportunities in the field of self-assembly and STM.
Keywords: external stimuli; phase transition; scanning tunneling microscopy; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chen H., Stoddart J. From Molecular to Supramolecular Electronics. Nat. Rev. Mater. 2021;6:804–828. doi: 10.1038/s41578-021-00302-2. - DOI
-
- Liu Y., Qiu X., Soni S., Chiechi R. Charge Transport through Molecular Ensembles: Recent Progress in Molecular Electronics. Chem. Phys. Rev. 2021;2:021303. doi: 10.1063/5.0050667. - DOI
-
- Zeng K., Tong Z., Ma L., Zhu W.-H., Wu W., Xie Y. Molecular Engineering Strategies for Fabricating Efficient Porphyrin-Based Dye-Sensitized Solar Cells. Energy Environ. Sci. 2020;13:1617–1657. doi: 10.1039/C9EE04200H. - DOI
-
- Huang X., Li T. Recent Progress in the Development of Molecular-Scale Electronics Based on Photoswitchable Molecules. J. Mater. Chem. C. 2020;8:821–848. doi: 10.1039/C9TC06054E. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

