Strategies in the Design and Development of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
- PMID: 37896769
- PMCID: PMC10610861
- DOI: 10.3390/v15101992
Strategies in the Design and Development of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
Abstract
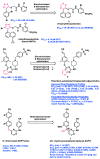
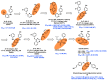
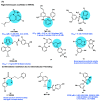
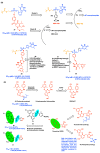
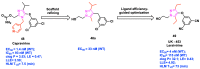
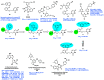
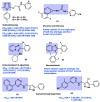
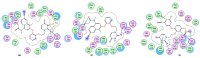
AIDS (acquired immunodeficiency syndrome) is a potentially life-threatening infectious disease caused by human immunodeficiency virus (HIV). To date, thousands of people have lost their lives annually due to HIV infection, and it continues to be a big public health issue globally. Since the discovery of the first drug, Zidovudine (AZT), a nucleoside reverse transcriptase inhibitor (NRTI), to date, 30 drugs have been approved by the FDA, primarily targeting reverse transcriptase, integrase, and/or protease enzymes. The majority of these drugs target the catalytic and allosteric sites of the HIV enzyme reverse transcriptase. Compared to the NRTI family of drugs, the diverse chemical class of non-nucleoside reverse transcriptase inhibitors (NNRTIs) has special anti-HIV activity with high specificity and low toxicity. However, current clinical usage of NRTI and NNRTI drugs has limited therapeutic value due to their adverse drug reactions and the emergence of multidrug-resistant (MDR) strains. To overcome drug resistance and efficacy issues, combination therapy is widely prescribed for HIV patients. Combination antiretroviral therapy (cART) includes more than one antiretroviral agent targeting two or more enzymes in the life cycle of the virus. Medicinal chemistry researchers apply different optimization strategies including structure- and fragment-based drug design, prodrug approach, scaffold hopping, molecular/fragment hybridization, bioisosterism, high-throughput screening, covalent-binding, targeting highly hydrophobic channel, targeting dual site, and multi-target-directed ligand to identify and develop novel NNRTIs with high antiviral activity against wild-type (WT) and mutant strains. The formulation experts design various delivery systems with single or combination therapies and long-acting regimens of NNRTIs to improve pharmacokinetic profiles and provide sustained therapeutic effects.
Keywords: HIV/AIDS; NNRTIs; bioisosteric replacement; cART; long-acting injectable; molecular hybridization; pharmaceutical strategies; prodrug; reverse transcriptase; scaffold hopping; structure- and fragment-based drug design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Current status of the non-nucleoside reverse transcriptase inhibitors of human immunodeficiency virus type 1.Curr Top Med Chem. 2004;4(9):921-44. doi: 10.2174/1568026043388420. Curr Top Med Chem. 2004. PMID: 15134549 Review.
-
Current insights and molecular docking studies of HIV-1 reverse transcriptase inhibitors.Chem Biol Drug Des. 2024 Jan;103(1):e14372. doi: 10.1111/cbdd.14372. Epub 2023 Oct 10. Chem Biol Drug Des. 2024. PMID: 37817296 Review.
-
Characteristics of a group of nonnucleoside reverse transcriptase inhibitors with structural diversity and potent anti-human immunodeficiency virus activity.Leukemia. 1995 Oct;9 Suppl 1:S75-85. Leukemia. 1995. PMID: 7475321
-
Combining New Non-Nucleoside Reverse Transcriptase Inhibitors (RTIs) with AZT Results in Strong Synergism against Multi-RTI-Resistant HIV-1 Strains.Molecules. 2018 Jul 2;23(7):1599. doi: 10.3390/molecules23071599. Molecules. 2018. PMID: 30004408 Free PMC article.
-
Development of non-nucleoside reverse transcriptase inhibitors (NNRTIs): our past twenty years.Acta Pharm Sin B. 2020 Jun;10(6):961-978. doi: 10.1016/j.apsb.2019.11.010. Epub 2019 Nov 21. Acta Pharm Sin B. 2020. PMID: 32642405 Free PMC article. Review.
Cited by
-
Mapping the Gut Microbiota Composition in the Context of Raltegravir, Dolutegravir, and Bictegravir-A Scoping Review.Int J Mol Sci. 2025 Jul 2;26(13):6366. doi: 10.3390/ijms26136366. Int J Mol Sci. 2025. PMID: 40650144 Free PMC article.
-
Design, Synthesis, and Biological Activity of Amine-Type Cyclopentanepyridinone Derivatives as HIV‑1 Non-Nucleoside Reverse Transcriptase Inhibitors.ACS Omega. 2025 Jul 18;10(29):32148-32160. doi: 10.1021/acsomega.5c03953. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757284 Free PMC article.
-
Identification of a novel small-molecule inhibitor of the HIV-1 reverse transcriptase activity with a non-nucleoside mode of action.Virol J. 2025 Mar 7;22(1):65. doi: 10.1186/s12985-025-02680-3. Virol J. 2025. PMID: 40055750 Free PMC article.
-
Efficacy assessment of antiretroviral drugs against equine infectious anemia virus in vitro.Virus Res. 2024 Dec;350:199503. doi: 10.1016/j.virusres.2024.199503. Epub 2024 Dec 11. Virus Res. 2024. PMID: 39613191 Free PMC article.
References
-
- Quan Y., Rong L., Liang C., Wainberg M.A. Reverse Transcriptase Inhibitors Can Selectively Block the Synthesis of Differently Sized Viral DNA Transcripts in Cells Acutely Infected with Human Immunodeficiency Virus Type 1. J. Virol. 1999;73:6700–6707. doi: 10.1128/JVI.73.8.6700-6707.1999. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

