Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis
- PMID: 37896803
- PMCID: PMC10611233
- DOI: 10.3390/v15102026
Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis
Abstract
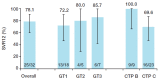
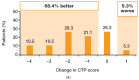
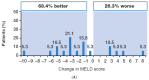
A fixed-dose combination of sofosbuvir/velpatasvir (SOF/VEL) plus weight-based ribavirin (RBV) for 12 weeks is recommended for the treatment of patients with hepatitis C virus (HCV)-associated decompensated cirrhosis. However, large global studies, while confirming the effectiveness of SOF/VEL in a broad range of patients, often exclude these patients. This Phase 2, single-arm, open-label study in adult patients with HCV-associated decompensated cirrhosis in France and the USA aimed to provide further data on the safety and efficacy of SOF/VEL plus RBV for 12 weeks in this population. Patients were treated with a fixed-dose combination of SOF 400 mg/VEL 100 mg plus weight-based RBV once daily for 12 weeks. The inclusion criteria were chronic HCV infection (≥6 months), quantifiable HCV RNA at screening, Child-Turcotte-Pugh class B or C cirrhosis, and liver imaging within 6 months of Day 1 to exclude hepatocellular carcinoma. Among 32 patients who initiated treatment, 78.1% achieved sustained virologic response 12 weeks after the end of treatment (SVR12). Failure to achieve SVR12 was due to non-virologic reasons (investigator discretion, n = 1; death, n = 6). All 25 patients in the per-protocol population achieved SVR12 and all but one achieved sustained virologic response 24 weeks after the end of treatment. Adverse events (AEs) were as expected for a patient population with advanced liver disease. All Grade 3-4 and serious AEs and deaths were deemed unrelated to treatment. In patients with HCV-associated decompensated cirrhosis, SOF/VEL plus RBV achieved high SVR12 rates and was generally well tolerated.
Keywords: HCV; decompensated cirrhosis; ribavirin; sofosbuvir/velpatasvir; sustained virologic response.
Conflict of interest statement
Steven Flamm has received honoraria for presentations from Gilead Sciences and AbbVie. Eric Lawitz has received grant/research support from 89Bio Inc., AbbVie, Akero Therapeutics, Allergan, Alnylam Pharmaceuticals Inc., Amgen, Ascelia Pharma, Assemblybio, Astrazeneca, Axcella Health, Biocryst Pharmaceuticals, Bird Rock Bio Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Conatus Pharmaceuticals, Cymabay Therapeutics, CytoDyn, DSM, Durect Corporation, Eli Lilly and Company, Enanta Pharmaceuticals, Enyo Pharma, Exalenz Bioscience, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Genentech, Gilead Sciences, GlaxoSmithKline, Hanmi Pharmaceuticals, Hightide Biopharma, Intercept Pharmaceuticals, Inventiva, Janssen Pharmaceuticals, Laboratory for Advanced Medicine, Loxo Oncology, Madrigal Pharmaceuticals, Merck & Co, Metacrine, NGM Biopharmaceuticals Inc., Northsea Therapeutics, Novartis, Novo Nordisk Inc., Pfizer, Poxel, Roche, Sagimet Biosciences, Synlogic Therapeutics, Terns Pharmaceuticals, Viking Therapeutics and Zydus Pharmaceuticals. He has also acted as a consultant for Akero, Boehringer Ingelheim, Bristol-Myers Squibb, Intercept, Novo Nordisk, Metacrine, Sagimet and Terns; and has received honoraria for presentations and manuscripts from Gilead Sciences, Abbvie, Intercept Akero, Boehringer Ingelheim, Bristol-Myers Squibb, Intercept, Novo Nordisk, Metacrine, Sagimet and Terns. Brian Borg has received grant/research support from Gilead Sciences. Michael Charlton has received grant/research support and has participated in a data safety monitoring board for Gilead Sciences. He has also acted as a consultant for AbbVie, Bristol-Myers Squibb and Pfizer. Charles Landis has received grant/research support from AbbVie, Gilead Sciences and Hightide. K Rajender Reddy has received grant/research support from Gilead Sciences, Bristol-Myers Squibb, Merck & Co, Intercept, Sequana, Grifols, Mallinckrodt, Exact Sciences, HCC-TARGET, NASH-TARGET, BioVie; has received royalties/licenses from UpToDate; and has has acted as a consultant for Nov Nordisk, Spark Therapeutics, Genfit, BioVie and Mallinckrodt. Mitchell Shiffman has received grant/research support and honoraria payments from AbbVie, Gilead Sciences. Natarajan Ravendhran has received honoraria for presentations and grant/research support from Gilead Sciences. Didier Samuel has acted as a consultant for Biotest and Go Liver. Candido Hernandez, Christophe Hézode, Stacey Scherbakovsky and Renee-Claude Mercier are all employees of Gilead Sciences. Angel Alsina and Charissa Chang have nothing to disclose.
Figures


References
-
- World Health Organization Hepatitis C Fact Sheet. [(accessed on 25 July 2023)]; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
-
- American Association for the Study of Liver Diseases HCV Guidance: Recommendations for Testing, Managing and Treating Hepatitis C. [(accessed on 25 July 2023)]. Available online: www.hcvguidelines.org.
-
- Takehara T., Sakamoto N., Nishiguchi S., Ikeda F., Tatsumi T., Ueno Y., Yatsuhashi H., Takikawa Y., Kanda T., Sakamoto M., et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: An open-label Phase 3 trial. J. Gastroenterol. 2019;54:87–95. doi: 10.1007/s00535-018-1503-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

