Immunogenicity and Safety of MF59-Adjuvanted Quadrivalent Influenza Vaccine Compared with a Nonadjuvanted, Quadrivalent Influenza Vaccine in Adults 50-64 Years of Age
- PMID: 37896932
- PMCID: PMC10611124
- DOI: 10.3390/vaccines11101528
Immunogenicity and Safety of MF59-Adjuvanted Quadrivalent Influenza Vaccine Compared with a Nonadjuvanted, Quadrivalent Influenza Vaccine in Adults 50-64 Years of Age
Abstract
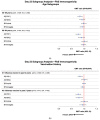
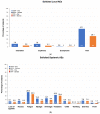
Adults aged 50-64 years have a high incidence of symptomatic influenza associated with substantial disease and economic burden each year. We conducted a randomized, controlled trial to compare the immunogenicity and safety of an adjuvanted quadrivalent inactivated influenza vaccine (aIIV4; n = 1027) with a nonadjuvanted standard dose IIV4 (n = 1017) in this population. Immunogenicity was evaluated on Days 22, 181, and 271. On Day 22, upper limits (UL) of 95% confidence intervals (CI) for geometric mean titer (GMT) ratios (IIV4/aIIV4) were <1.5 and 95% CI ULs for the difference in seroconversion rate (SCR IIV4 - aIIV4) were <10% for all four vaccine strains, meeting primary endpoint noninferiority criteria. Protocol-defined superiority criteria (95% CI ULs < 1.0) were also met for A(H1N1) and A(H3N2). Immune responses following aIIV4 vaccination were more pronounced in persons with medical comorbidities and those not recently vaccinated against influenza. Safety data were consistent with previous studies of MF59 adjuvanted seasonal and pandemic influenza vaccines. These findings support the immunological benefit of aIIV4 for persons aged 50-64 years, especially those with comorbidities.
Keywords: adjuvanted influenza vaccine; age 50–64 years; chronic medical condition; influenza; middle-aged adults.
Conflict of interest statement
A.P, J.M. and P.H. report support for this trial funded by CSL Seqirus. J.O., D.M., E.V., L.I., Q.Z. and M.H. are employees of CSL Seqirus.
Figures


References
-
- Centers for Disease Control and Prevention Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2018–2019 Influenza Season. [(accessed on 3 July 2023)]; Available online: https://www.cdc.gov/flu/about/burden/2018-2019.html.
-
- Grohskopf L.A., Blanton L.H., Ferdinands J.M., Chung J.R., Broder K.R., Talbot H.K., Morgan R.L., Fry A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022–2023 Influenza Season. MMWR Recomm. Rep. 2022;71:1–28. doi: 10.15585/mmwr.rr7101a1. - DOI - PMC - PubMed
-
- UK Health and Security Agency Guidance: The Flu Vaccination: Who Should Have It and Why. [(accessed on 27 March 2023)]; Available online: https://www.gov.uk/government/publications/flu-vaccination-who-should-ha....
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

