Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens
- PMID: 37896988
- PMCID: PMC10611113
- DOI: 10.3390/vaccines11101585
Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens
Abstract
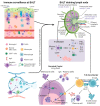
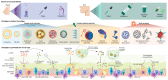
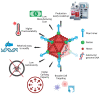
Inducing humoral and cytotoxic mucosal immunity at the sites of pathogen entry has the potential to prevent the infection from getting established. This is different from systemic vaccination, which protects against the development of systemic symptoms. The field of mucosal vaccination has seen fewer technological advances compared to nucleic acid and subunit vaccine advances for injectable vaccine platforms. The advent of the next-generation adenoviral vectors has given a boost to mucosal vaccine research. Basic research into the mechanisms regulating innate and adaptive mucosal immunity and the discovery of effective and safe mucosal vaccine adjuvants will continue to improve mucosal vaccine design. The results from clinical trials of inhaled COVID-19 vaccines demonstrate their ability to induce the proliferation of cytotoxic T cells and the production of secreted IgA and IgG antibodies locally, unlike intramuscular vaccinations. However, these mucosal vaccines induce systemic immune responses at par with systemic vaccinations. This review summarizes the function of the respiratory mucosa-associated lymphoid tissue and the advantages that the adenoviral vectors provide as inhaled vaccine platforms.
Keywords: COVID-19; SARS-CoV-2; adenovirus; influenza; inhaled vaccines; mRNA; mucosa-associated lymphoid tissue; mucosal immunity; respiratory infections.
Conflict of interest statement
The authors are employed by Ocugen Inc., which is developing inhaled mucosal vaccines.
Figures

References
-
- Sunagar R., Prasad S.D., Ella R., Vadrevu K.M. Preclinical evaluation of safety and immunogenicity of a primary series intranasal COVID-19 vaccine candidate (BBV154) and humoral immunogenicity evaluation of a heterologous prime-boost strategy with COVAXIN (BBV152) Front. Immunol. 2022;13:1063679. doi: 10.3389/fimmu.2022.1063679. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

