Comparative Cost-Effectiveness Analysis of Respiratory Syncytial Virus Vaccines for Older Adults in Hong Kong
- PMID: 37897008
- PMCID: PMC10610694
- DOI: 10.3390/vaccines11101605
Comparative Cost-Effectiveness Analysis of Respiratory Syncytial Virus Vaccines for Older Adults in Hong Kong
Abstract
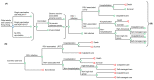
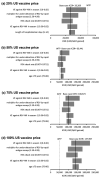
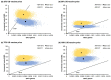
Two respiratory syncytial virus (RSV) vaccines (AREXVY® and ABRYSVO®) were recently approved for older adults in the US. This study aimed to evaluate the cost-effectiveness of AREXVY® and ABRYSVO® from the Hong Kong public healthcare provider's perspective. A two-year decision-analytical model was developed to examine the outcomes of a single RSV vaccination (AREXVY® or ABRYSVO®) compared to no vaccination. Primary outcomes included RSV-related health outcomes, direct medical costs, quality-adjusted life-year (QALY) loss, and incremental cost per QALY (ICER). RSV vaccines are not yet marketed in Hong Kong, base-case analysis, therefore, benchmarked US RSV vaccine prices at 4 levels (25%, 50%, 75%, 100%). AREXVY® and ABRYSVO® (versus no vaccination) gained 0.000568 QALY and 0.000647 QALY, respectively. ICERs of ABRYSVO® (26,209 USD/QALY) and AREXVY® (47,485 USD/QALY) were lower than the willingness-to-pay threshold (49,594 USD/QALY) at 25% US vaccine price. The RSV attack rate was a common influential factor at all vaccine price levels. The probabilities of AREXVY® and ABRYSVO® to be most cost-effective were 0.10% and 97.68%, respectively, at 25% US vaccine price. Single vaccination of ABRYSVO® or AREXVY® for older adults appears to gain QALYs over 2 years in Hong Kong. The cost-effectiveness of AREXVY® and ABRYSVO® is subject to vaccine price and RSV attack rate.
Keywords: cost-effectiveness analysis; older adults; respiratory infection; respiratory syncytial virus; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Shi T., Denouel A., Tietjen A.K., Campbell I., Moran E., Li X., Campbell H., Demont C., Nyawanda B.O., Chu H.Y., et al. Global Disease Burden Estimates of Respiratory Syncytial Virus–Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020;222:S577–S583. doi: 10.1093/infdis/jiz059. - DOI - PubMed
-
- Zhang Y., Wang Y., Zhao J., Xiong Z., Fan Y., Zhang W., Zou X., Wang C., Han J., Li B., et al. Severity and mortality of respiratory syncytial virus vs influenza A infection in hospitalized adults in China. Influenza Other Respi. Viruses. 2020;14:483–490. doi: 10.1111/irv.12754. - DOI - PMC - PubMed
-
- Chan P.K.S., Tam W.W.S., Lee T.C., Hon K.L., Lee N., Chan M.C.W., Mok H.Y., Wong M.C.S., Leung T.F., Lai R.W.M., et al. Hospitalization Incidence, Mortality, and Seasonality of Common Respiratory Viruses Over a Period of 15 Years in a Developed Subtropical City. Medicine. 2015;94:e2024. doi: 10.1097/MD.0000000000002024. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

