Effectiveness of Cell-Based Quadrivalent Seasonal Influenza Vaccine: A Systematic Review and Meta-Analysis
- PMID: 37897009
- PMCID: PMC10610589
- DOI: 10.3390/vaccines11101607
Effectiveness of Cell-Based Quadrivalent Seasonal Influenza Vaccine: A Systematic Review and Meta-Analysis
Abstract
Cell-based seasonal influenza vaccine viruses may more closely match recommended vaccine strains than egg-based options. We sought to evaluate the effectiveness of seasonal cell-based quadrivalent influenza vaccine (QIVc), as reported in the published literature. A systematic literature review was conducted (PROSPERO CRD42020160851) to identify publications reporting on the effectiveness of QIVc in persons aged ≥6 months relative to no vaccination or to standard-dose, egg-based quadrivalent or trivalent influenza vaccines (QIVe/TIVe). Publications from between 1 January 2016 and 25 February 2022 were considered. The review identified 18 relevant publications spanning three influenza seasons from the 2017-2020 period, with an overall pooled relative vaccine effectiveness (rVE) of 8.4% (95% CI, 6.5-10.2%) for QIVc vs. QIVe/TIVe. Among persons aged 4-64 years, the pooled rVE was 16.2% (95% CI, 7.6-24.8%) for 2017-2018, 6.1% (4.9-7.3%) for 2018-2019, and 10.1% (6.3-14.0%) for 2019-2020. For adults aged ≥65 years, the pooled rVE was 9.9% (95% CI, 6.9-12.9%) in the egg-adapted 2017-2018 season, whereas there was no significant difference in 2018-2019. For persons aged 4-64 years, QIVc was consistently more effective than QIVe/TIVe over the three influenza seasons. For persons aged ≥65 years, protection with QIVc was greater than QIVe or TIVe during the 2017-2018 season and comparable in 2018-2019.
Keywords: cell-based; influenza; quadrivalent influenza vaccine; vaccine effectiveness.
Conflict of interest statement
I.M. and M.H. are employed by CSL Seqirus Inc. B.L.C. and I.G. report receipt of funding to their institution (Sinai Health) from CSL Seqirus Inc.
Figures
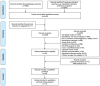
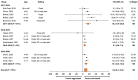

References
-
- Zost S.J., Parkhouse K., Gumina M.E., Kim K., Diaz Perez S., Wilson P.C., Treanor J.J., Sant A.J., Cobey S., Hensley S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA. 2017;114:12578–12583. doi: 10.1073/pnas.1712377114. - DOI - PMC - PubMed
-
- de Lejarazu-Leonardo O.R., Montomoli E., Wojcik R., Christopher S., Mosnier A., Pariani E., Trilla Garcia A., Fickenscher H., Gärtner B.C., Jandhyala R., et al. Estimation of reduction in influenza vaccine effectiveness due to egg-adaptation changes—Systematic literature review and expert consensus. Vaccines. 2021;9:1255. doi: 10.3390/vaccines9111255. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

