Human HELQ regulates DNA end resection at DNA double-strand breaks and stalled replication forks
- PMID: 37897354
- PMCID: PMC10711563
- DOI: 10.1093/nar/gkad940
Human HELQ regulates DNA end resection at DNA double-strand breaks and stalled replication forks
Abstract
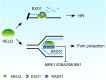
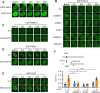
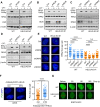
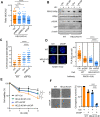
Following a DNA double strand break (DSB), several nucleases and helicases coordinate to generate single-stranded DNA (ssDNA) with 3' free ends, facilitating precise DNA repair by homologous recombination (HR). The same nucleases can act on stalled replication forks, promoting nascent DNA degradation and fork instability. Interestingly, some HR factors, such as CtIP and BRCA1, have opposite regulatory effects on the two processes, promoting end resection at DSB but inhibiting the degradation of nascent DNA on stalled forks. However, the reason why nuclease actions are regulated by different mechanisms in two DNA metabolism is poorly understood. We show that human HELQ acts as a DNA end resection regulator, with opposing activities on DNA end resection at DSBs and on stalled forks as seen for other regulators. Mechanistically, HELQ helicase activity is required for EXO1-mediated DSB end resection, while ssDNA-binding capacity of HELQ is required for its recruitment to stalled forks, facilitating fork protection and preventing chromosome aberrations caused by replication stress. Here, HELQ synergizes with CtIP but not BRCA1 or BRCA2 to protect stalled forks. These findings reveal an unanticipated role of HELQ in regulating DNA end resection at DSB and stalled forks, which is important for maintaining genome stability.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Aguilera A., Gomez-Gonzalez B.. Genome instability: a mechanistic view of its causes and consequences. Nat. Rev. Genet. 2008; 9:204–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

