Mapping of neuronal and glial primary cilia contactome and connectome in the human cerebral cortex
- PMID: 37898123
- PMCID: PMC10841524
- DOI: 10.1016/j.neuron.2023.09.032
Mapping of neuronal and glial primary cilia contactome and connectome in the human cerebral cortex
Abstract
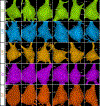
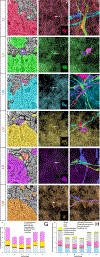
Primary cilia act as antenna receivers of environmental signals and enable effective neuronal or glial responses. Disruption of their function is associated with circuit disorders. To understand the signals these cilia receive, we comprehensively mapped cilia's contacts within the human cortical connectome using serial-section EM reconstruction of a 1 mm3 cortical volume, spanning the entire cortical thickness. We mapped the "contactome" of cilia emerging from neurons and astrocytes in every cortical layer. Depending on the layer and cell type, cilia make distinct patterns of contact. Primary cilia display cell-type- and layer-specific variations in size, shape, and microtubule axoneme core, which may affect their signaling competencies. Neuronal cilia are intrinsic components of a subset of cortical synapses and thus a part of the connectome. This diversity in the structure, contactome, and connectome of primary cilia endows each neuron or glial cell with a unique barcode of access to the surrounding neural circuitry.
Keywords: ciliopathies; connectome; cortical circuit; human brain disorders; primary cilia; synapse.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
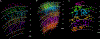





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

