Adherence, safety, and choice of the monthly dapivirine vaginal ring or oral emtricitabine plus tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis among African adolescent girls and young women: a randomised, open-label, crossover trial
- PMID: 37898146
- PMCID: PMC10756058
- DOI: 10.1016/S2352-3018(23)00227-8
Adherence, safety, and choice of the monthly dapivirine vaginal ring or oral emtricitabine plus tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis among African adolescent girls and young women: a randomised, open-label, crossover trial
Abstract
Background: Half of new HIV acquisitions in Africa occur in adolescent girls and young women. Pre-exposure prophylaxis (PrEP) with oral tenofovir disoproxil fumarate plus emtricitabine or the monthly dapivirine vaginal ring is efficacious but has lower adherence and effectiveness among adolescent girls and young women. We aimed to assess product adherence, safety, and choice of oral PrEP compared with the dapivirine ring among African adolescent girls and young women.
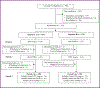
Methods: MTN-034/REACH was a randomised, open-label, phase 2a crossover trial among HIV-seronegative, non-pregnant adolescent girls and young women aged 16-21 years at four clinical research sites in South Africa, Uganda, and Zimbabwe. Participants were randomly assigned (1:1) to either the dapivirine ring or daily oral PrEP (200 mg of emtricitabine and 300 mg of tenofovir disoproxil fumarate) for 6 months, then switched to the other product option for 6 months, followed by a third 6-month period in which participants were given a choice of oral PrEP, the dapivirine ring, or neither. Fixed block randomisation was used, stratified by site. The primary adherence endpoint was use of each product during the randomised periods, with high use defined as tenofovir-diphosphate concentrations greater than or equal to 700 fmol/punch (associated with taking an average of four or more tablets per week in the previous month) and greater than or equal to 4 mg dapivirine released from the returned ring (continuous use for 28 days in the previous month) based on residual drug concentrations. The primary safety endpoint was grade 2 or higher adverse events during each randomised period of 24 weeks of ring and oral PrEP. This trial is registered at ClinicalTrials.gov, NCT03593655.
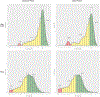
Findings: From Feb 6, 2019 to Sept 9, 2021, 396 adolescent girls and young women were screened, 247 of whom were enrolled and randomly assigned (6 months of the ring followed by 6 months of oral PrEP n=124; 6 months of oral PrEP followed by 6 months of the ring n=123). Median age was 18 years (IQR 17-19). 54 grade 2 or higher product-related adverse events were reported during oral PrEP and five during dapivirine ring use, with no product-related serious adverse events. High adherence was observed in 753 (57%) of the 1316 oral PrEP visits and 806 (57%) of the 1407 dapivirine ring visits. Four women acquired HIV during follow-up.
Interpretation: Adherence was moderately high and similar between oral PrEP and the dapivirine ring with favourable safety and tolerability. Oral PrEP and the dapivirine ring are effective, safe, and well tolerated HIV prevention options for adolescent girls and young women who would benefit from a choice of PrEP formulations to meet their needs and preferences.
Funding: National Institutes of Health.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CC has received consulting fees from Gilead Sciences and Merck, and has been an expert witness for Gilead. SLH has received consulting fees and funds to her institution from Merck. KN has received research funds from Merck (Merck Sharpe & Dohme). JMB and JFR are employees of Gilead Sciences. All other authors declare no competing interests.
Figures
Comment in
-
PrEParing for choice in a new era of HIV prevention.Lancet HIV. 2023 Dec;10(12):e757-e758. doi: 10.1016/S2352-3018(23)00263-1. Epub 2023 Oct 25. Lancet HIV. 2023. PMID: 37898147 Free PMC article. No abstract available.
References
-
- Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

