Mitochondrial hydrogen peroxide production by pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in oxidative eustress and oxidative distress
- PMID: 37898400
- PMCID: PMC10692731
- DOI: 10.1016/j.jbc.2023.105399
Mitochondrial hydrogen peroxide production by pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in oxidative eustress and oxidative distress
Abstract
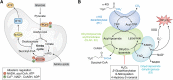
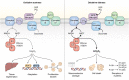
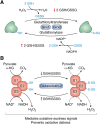
Pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (KGDH) are vital entry points for monosaccharides and amino acids into the Krebs cycle and thus integral for mitochondrial bioenergetics. Both complexes produce mitochondrial hydrogen peroxide (mH2O2) and are deactivated by electrophiles. Here, we provide an update on the role of PDH and KGDH in mitochondrial redox balance and their function in facilitating metabolic reprogramming for the propagation of oxidative eustress signals in hepatocytes and how defects in these pathways can cause liver diseases. PDH and KGDH are known to account for ∼45% of the total mH2O2 formed by mitochondria and display rates of production several-fold higher than the canonical source complex I. This mH2O2 can also be formed by reverse electron transfer (RET) in vivo, which has been linked to metabolic dysfunctions that occur in pathogenesis. However, the controlled emission of mH2O2 from PDH and KGDH has been proposed to be fundamental for oxidative eustress signal propagation in several cellular contexts. Modification of PDH and KGDH with protein S-glutathionylation (PSSG) and S-nitrosylation (PSNO) adducts serves as a feedback inhibitor for mH2O2 production in response to glutathione (GSH) pool oxidation. PSSG and PSNO adduct formation also reprogram the Krebs cycle to generate metabolites vital for interorganelle and intercellular signaling. Defects in the redox modification of PDH and KGDH cause the over generation of mH2O2, resulting in oxidative distress and metabolic dysfunction-associated fatty liver disease (MAFLD). In aggregate, PDH and KGDH are essential platforms for emitting and receiving oxidative eustress signals.
Keywords: fatty liver disease; hydrogen peroxide; mitochondria; oxidative distress; oxidative eustress; pyruvate dehydrogenase; redox signaling; α-ketoglutarate dehydrogenase.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Targeted Redox Regulation α-Ketoglutarate Dehydrogenase Complex for the Treatment of Human Diseases.Cells. 2025 Apr 29;14(9):653. doi: 10.3390/cells14090653. Cells. 2025. PMID: 40358176 Free PMC article. Review.
-
Fatty acid oxidation drives mitochondrial hydrogen peroxide production by α-ketoglutarate dehydrogenase.J Biol Chem. 2024 Apr;300(4):107159. doi: 10.1016/j.jbc.2024.107159. Epub 2024 Mar 11. J Biol Chem. 2024. PMID: 38479602 Free PMC article.
-
Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex.Free Radic Biol Med. 2017 May;106:302-314. doi: 10.1016/j.freeradbiomed.2017.02.046. Epub 2017 Feb 27. Free Radic Biol Med. 2017. PMID: 28242228
-
S-nitroso-glutathione (GSNO) inhibits hydrogen peroxide production by alpha-ketoglutarate dehydrogenase: An investigation into sex and diet effects.Free Radic Biol Med. 2023 Aug 1;204:287-300. doi: 10.1016/j.freeradbiomed.2023.05.010. Epub 2023 May 22. Free Radic Biol Med. 2023. PMID: 37225107
-
α-Ketoglutarate dehydrogenase: a mitochondrial redox sensor.Free Radic Res. 2011 Jan;45(1):29-36. doi: 10.3109/10715762.2010.534163. Epub 2010 Nov 29. Free Radic Res. 2011. PMID: 21110783 Free PMC article. Review.
Cited by
-
Ferroptosis: iron release mechanisms in the bioenergetic process.Cancer Metastasis Rev. 2025 Feb 25;44(1):36. doi: 10.1007/s10555-025-10252-8. Cancer Metastasis Rev. 2025. PMID: 40000477 Review.
-
Targeted Redox Regulation α-Ketoglutarate Dehydrogenase Complex for the Treatment of Human Diseases.Cells. 2025 Apr 29;14(9):653. doi: 10.3390/cells14090653. Cells. 2025. PMID: 40358176 Free PMC article. Review.
-
Redox organization of living systems.Free Radic Biol Med. 2024 May 1;217:179-189. doi: 10.1016/j.freeradbiomed.2024.03.008. Epub 2024 Mar 14. Free Radic Biol Med. 2024. PMID: 38490457 Free PMC article. Review.
-
Enhancing mitophagy by ligustilide through BNIP3-LC3 interaction attenuates oxidative stress-induced neuronal apoptosis in spinal cord injury.Int J Biol Sci. 2024 Aug 12;20(11):4382-4406. doi: 10.7150/ijbs.98051. eCollection 2024. Int J Biol Sci. 2024. PMID: 39247814 Free PMC article.
-
In depth profiling of dihydrolipoamide dehydrogenase deficiency in primary patients fibroblasts reveals metabolic reprogramming secondary to mitochondrial dysfunction.Mol Genet Metab Rep. 2024 Dec 16;42:101172. doi: 10.1016/j.ymgmr.2024.101172. eCollection 2025 Mar. Mol Genet Metab Rep. 2024. PMID: 39802097 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

