The universe of galectin-binding partners and their functions in health and disease
- PMID: 37898403
- PMCID: PMC10696404
- DOI: 10.1016/j.jbc.2023.105400
The universe of galectin-binding partners and their functions in health and disease
Abstract
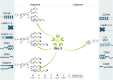
Galectins, a family of evolutionarily conserved glycan-binding proteins, play key roles in diverse biological processes including tissue repair, adipogenesis, immune cell homeostasis, angiogenesis, and pathogen recognition. Dysregulation of galectins and their ligands has been observed in a wide range of pathologic conditions including cancer, autoimmune inflammation, infection, fibrosis, and metabolic disorders. Through protein-glycan or protein-protein interactions, these endogenous lectins can shape the initiation, perpetuation, and resolution of these processes, suggesting their potential roles in disease monitoring and treatment. However, despite considerable progress, a full understanding of the biology and therapeutic potential of galectins has not been reached due to their diversity, multiplicity of cell targets, and receptor promiscuity. In this article, we discuss the multiple galectin-binding partners present in different cell types, focusing on their contributions to selected physiologic and pathologic settings. Understanding the molecular bases of galectin-ligand interactions, particularly their glycan-dependency, the biochemical nature of selected receptors, and underlying signaling events, might contribute to designing rational therapeutic strategies to control a broad range of pathologic conditions.
Keywords: angiogenesis; galectins; glycoproteins; glycosylation; immunity; receptors; tumorigenesis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest M. F. T., M. T. E., A. G. B., L. S. and M. V. E. declare that they have no conflict of interest regarding the content of this article. G. A. R. reports a relationship with Galtec SAS that includes equities.
Figures





References
-
- Yang R.Y., Rabinovich G.A., Liu F.T. Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008;10:1–24. - PubMed
-
- Thiemann S., Baum L.G. Galectins and immune responses-just how do they do those things they do? Annu. Rev. Immunol. 2016;34:243–264. - PubMed
-
- Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta. 2002;1572:232–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

