Are maternal vaccines effective and safe for mothers and infants? A systematic review and meta-analysis of randomised controlled trials
- PMID: 37899087
- PMCID: PMC10619060
- DOI: 10.1136/bmjgh-2023-012376
Are maternal vaccines effective and safe for mothers and infants? A systematic review and meta-analysis of randomised controlled trials
Abstract
Introduction: Maternal vaccination is a promising strategy to reduce the burden of vaccine-preventable diseases for mothers and infants. We aimed to provide an up-to-date overview of the efficacy and safety of all available maternal vaccines.
Methods: We searched PubMed, Embase, CENTRAL and ClinicalTrials.gov on 1 February 2022, for phase III and IV randomised controlled trials (RCTs) that compared maternal vaccination against any pathogen with placebo or no vaccination. Primary outcomes were laboratory-confirmed or clinically confirmed disease in mothers and infants. Secondary safety outcomes included intrauterine growth restriction, stillbirth, maternal death, preterm birth, congenital malformations and infant death. Random effects meta-analysis were used to calculate pooled risk ratio's (RR). Quality appraisal was performed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE).
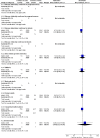
Results: Six RCTs on four maternal vaccines, influenza, tetanus, diphtheria and pertussis (Tdap), pneumococcal and respiratory syncytial virus (RSV) were eligible. The overall risk of bias and certainty of evidence varied from low to high. Maternal influenza vaccination significantly reduced the number of laboratory-confirmed influenza cases (RR 0.58, 95% CI 0.42 to 0.79, event rate 57 vs 98, 2 RCTs, n=6003, I2=0%), and clinically confirmed influenza cases in mothers (RR 0.88, 95% CI 0.78 to 0.99, event rate 418 vs 472, 2 RCTs, n=6003, I2=0%), and laboratory-confirmed influenza in infants (RR 0.66, 95% CI 0.52 to 0.85, event rate 98 vs 148, 2 RCTs, n=5883, I2=0%), although this was not significant for clinically confirmed influenza in infants (RR 0.99, 95% CI 0.94 to 1.05, event rate 1371 vs 1378, 2 RCTs, n=5883, I2=0%). No efficacy data were available on maternal Tdap vaccination. Maternal pneumococcal vaccination did not reduce laboratory-confirmed and clinically confirmed middle ear disease (RR 0.49, 95% CI 0.24 to 1.02, event rate 9 vs 18, 1 RCT, n=133 and RR 0.88 95% CI 0.69 to 1.12, event rate 42 vs 47, 1 RCT, n=133, respectively), and clinically confirmed lower-respiratory tract infection (LRTI) (RR 1.08, 95% CI 0.82 to 1.43, event rate 18 vs 34, 1 RCT, n=70) in infants. Maternal RSV vaccination did not reduce laboratory-confirmed RSV LRTI in infants (RR 0.75, 95% CI 0.56 to 1.01, event rate 103 vs 71, 1 RCT, n=4527). There was no evidence of a significant effect of any of the maternal vaccines on the reported safety outcomes.
Conclusions: The few RCTs with low event rates suggest that, depending on the type of maternal vaccine, the vaccine might effectively prevent disease and within its size does not show safety concerns in mothers and infants.
Prospero registration number: CRD42021235115.
Keywords: Child health; Immunisation; Maternal health; Systematic review; Vaccines.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JW participated in the advisory board of Janssen with fees paid to UMCU. MS leads a department that conducts studies on COVID-19 vaccines for the European Medicines Agency, Pfizer, AstraZeneca and Janssen. All according to the ENCePP code of conduct. KB is principal investigator of a phase 3 clinical trial on maternal RSV vaccination funded by Pfizer and of work package three of the Consign study funded by EMA. LJB has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. UMCU has received major funding (>€100 000 per industrial partner) for investigator initiated studies from AbbVie, MedImmune, Janssen, the Bill and Melinda Gates Foundation, Nutricia (Danone) and MeMed Diagnostics. UMCU has received major cash or in kind funding as part of the public private partnership IMI-funded RESCEU project from GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi. UMCU has received major funding by Julius Clinical for participating in the INFORM study sponsored by MedImmune. UMCU has received minor funding for participation in trials by Regeneron and Janssen from 2015 to 2017 (total annual estimate less than €20 000). UMCU received minor funding for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, Novavax, Pfizer, Janssen (total annual estimate less than €20 000). LJB is the founding chairman of the ReSViNET Foundation. All other authors have nothing to disclose (OB, EP, FA and NvdM).
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
