Patient-derived lymphoma spheroids integrating immune tumor microenvironment as preclinical follicular lymphoma models for personalized medicine
- PMID: 37899130
- PMCID: PMC10619028
- DOI: 10.1136/jitc-2023-007156
Patient-derived lymphoma spheroids integrating immune tumor microenvironment as preclinical follicular lymphoma models for personalized medicine
Abstract
Background: Follicular lymphoma (FL), the most common indolent non-Hodgkin's Lymphoma, is a heterogeneous disease and a paradigm of the contribution of immune tumor microenvironment to disease onset, progression, and therapy resistance. Patient-derived models are scarce and fail to reproduce immune phenotypes and therapeutic responses.
Methods: To capture disease heterogeneity and microenvironment cues, we developed a patient-derived lymphoma spheroid (FL-PDLS) model culturing FL cells from lymph nodes (LN) with an optimized cytokine cocktail that mimics LN stimuli and maintains tumor cell viability.
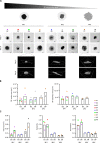
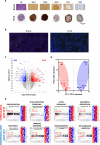
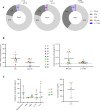
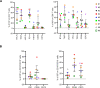
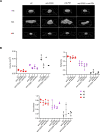
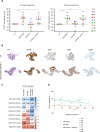
Results: FL-PDLS, mainly composed of tumor B cells (60% on average) and autologous T cells (13% CD4 and 3% CD8 on average, respectively), rapidly organizes into patient-specific three-dimensional (3D) structures of three different morphotypes according to 3D imaging analysis. RNAseq analysis indicates that FL-PDLS reproduces FL hallmarks with the overexpression of cell cycle, BCR, or mTOR signaling related gene sets. FL-PDLS also recapitulates the exhausted immune phenotype typical of FL-LN, including expression of BTLA, TIGIT, PD-1, TIM-3, CD39 and CD73 on CD3+ T cells. These features render FL-PDLS an amenable system for immunotherapy testing. With this aim, we demonstrate that the combination of obinutuzumab (anti-CD20) and nivolumab (anti-PD1) reduces tumor load in a significant proportion of FL-PDLS. Interestingly, B cell depletion inversely correlates with the percentage of CD8+ cells positive for PD-1 and TIM-3.
Conclusions: In summary, FL-PDLS is a robust patient-derived 3D system that can be used as a tool to mimic FL pathology and to test novel immunotherapeutic approaches in a context of personalized medicine.
Keywords: Hematologic Neoplasms; Immune Checkpoint Inhibitors; Immunotherapy; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RM and J-ML are employees of Imactiv3D.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
