Health-related quality of life and its determinants during and after treatment for paediatric acute lymphoblastic leukaemia: a national, prospective, longitudinal study in the Netherlands
- PMID: 37899146
- PMCID: PMC10619055
- DOI: 10.1136/bmjopen-2022-070804
Health-related quality of life and its determinants during and after treatment for paediatric acute lymphoblastic leukaemia: a national, prospective, longitudinal study in the Netherlands
Abstract
Objectives: Health-related quality of life (HRQoL) is impaired in paediatric patients with acute lymphoblastic leukaemia (ALL). Over the past decades, ALL treatment has successfully been adjusted to the risk of relapse, which is now reflected by the stratification of patients into three risk groups who receive treatment of differing intensities. This study is the first to evaluate the longitudinal course of HRQoL in light of these adjustments and identify determinants of HRQoL.
Design: Two prospective, national cohort studies (add-on studies within the two most recent treatment protocols for children with ALL (ALL-10 and ALL-11)).
Setting: Dutch paediatric oncology hospitals between October 2006 and October 2009 (ALL-10) and between August 2013 and July 2017 (ALL-11).
Participants: Patients with ALL (2-18 years) are treated according to the ALL-10 or ALL-11 treatment protocol. Patients treated according to the ALL-10 protocol only completed a cancer-specific QoL measure and patients treated according to the ALL-11 protocol completed both a cancer-specific and generic QoL measure (see below).
Outcome measures: HRQoL, assessed with parent-proxy questionnaires (PedsQL Generic and Cancer module) within the first 5 months (T0), at 1 year (T1), 2 years (T2) and 3 years (T3) after diagnosis. The proportion of patients with clinically relevant generic HRQoL impairment was compared with healthy norm values. Multivariable mixed model analyses were used to evaluate the development of HRQoL over time and its medical and sociodemographic determinants (collected on enrolment).
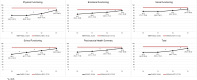
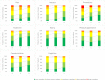
Results: Of the ALL-10 cohort, 132 families participated and of the ALL-11 cohort, 136 families participated (268 total). Thus, cancer-specific HRQoL assessments were available for 268 patients (median age 5.3 years (IQR 6.15), 56.0% boys, 69.0% medium-risk ALL), and generic HRQoL assessments for 136 patients (median age 4.8 years (IQR 6.13), 60.3% boys, 75.0% medium-risk ALL). Generic HRQoL improved between timepoints T0 and T3 (total score B 16.1, 95% CI 12.2 to 20.1, p<0.001), but did not restore to normal 1 year after the end of treatment: 28.0% of children remained impaired compared with 16% in the general population (p=0.003). Cancer-specific HRQoL generally improved from T0 to T2 (Pain B 11.3, 95% CI 7.1 to 15.5; Nausea B 11.7, 8.4 to 15.1; Procedural Anxiety B 19.1, 14.8 to 23.4; Treatment Anxiety B 12.8, 9.5 to 16.0; Worry B 3.5, 0.6 to 6.3; Communication B 8.5, 5.0 to 11.9; all p<0.001 except for Worry (p=0.02)), while Physical Appearance and Cognitive Functioning remained stable. Higher treatment intensity and experiencing pain or simultaneous chronic illness were associated with lower HRQoL over time for multiple subscales.
Conclusions: HRQoL impairment is prevalent during and after ALL treatment. Patients with standard-risk ALL and reduced treatment intensity have better HRQoL than patients in higher risk groups. Systematic monitoring of HRQoL is of utmost importance in order to provide timely psychosocial interventions and supportive care.
Keywords: Leukaemia; ONCOLOGY; Paediatric oncology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Prospective evaluation of quality of life in children treated in UKALL 2003 for acute lymphoblastic leukaemia: A cohort study.Pediatr Blood Cancer. 2017 Nov;64(11). doi: 10.1002/pbc.26615. Epub 2017 May 5. Pediatr Blood Cancer. 2017. PMID: 28475268 Clinical Trial.
-
Prospective longitudinal evaluation of treatment-related toxicity and health-related quality of life during the first year of treatment for pediatric acute lymphoblastic leukemia.BMC Cancer. 2022 Sep 15;22(1):985. doi: 10.1186/s12885-022-10072-x. BMC Cancer. 2022. PMID: 36109702 Free PMC article.
-
Effect of dexamethasone on quality of life in children with acute lymphoblastic leukaemia: a prospective observational study.Health Qual Life Outcomes. 2008 Nov 26;6:103. doi: 10.1186/1477-7525-6-103. Health Qual Life Outcomes. 2008. PMID: 19036151 Free PMC article.
-
Health-related quality of life in paediatric patients up to five years post-treatment completion for acute lymphoblastic leukaemia: a systematic review.Support Care Cancer. 2019 Nov;27(11):4341-4351. doi: 10.1007/s00520-019-04747-8. Epub 2019 Mar 21. Support Care Cancer. 2019. PMID: 30900055
-
Are We Agreed? Self- Versus Proxy-Reporting of Paediatric Health-Related Quality of Life (HRQoL) Using Generic Preference-Based Measures: A Systematic Review and Meta-Analysis.Pharmacoeconomics. 2022 Nov;40(11):1043-1067. doi: 10.1007/s40273-022-01177-z. Epub 2022 Aug 23. Pharmacoeconomics. 2022. PMID: 35997957 Free PMC article.
Cited by
-
Benchmarking the pediatric quality of life (PedsQL) cancer module in a large Dutch national cohort of childhood cancer patients.BMC Cancer. 2025 May 21;25(1):915. doi: 10.1186/s12885-025-14322-6. BMC Cancer. 2025. PMID: 40399814 Free PMC article.
-
The Impact of Thyroidectomy and Lobectomy on Patients' Health-Related Quality of Life, Eastern Region, Saudi Arabia.Clin Pract. 2024 Jun 29;14(4):1251-1263. doi: 10.3390/clinpract14040101. Clin Pract. 2024. PMID: 39051295 Free PMC article.
References
-
- DutchChildhoodOncologyGroup . SKION Jaarverslag. 2018. Available: https://www.skion.nl/workspace/uploads/Skion-Jaarverslag-2018_1.pdf
-
- Pieters R, de Groot-Kruseman H, Van der Velden V, et al. . Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study All10 from the dutch childhood oncology group. J Clin Oncol 2016;34:2591–601. 10.1200/JCO.2015.64.6364 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
