Spatially resolved CO2 carbon stable isotope analyses at the microscale using Raman spectroscopy
- PMID: 37899368
- PMCID: PMC10613625
- DOI: 10.1038/s41598-023-44903-z
Spatially resolved CO2 carbon stable isotope analyses at the microscale using Raman spectroscopy
Abstract
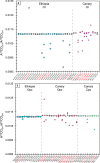
Measuring the carbon stable isotope ratio (13C/12C, expressed as δ13CCO2) in geogenic CO2 fluids is a crucial geochemical tool for studying Earth's degassing. Carbon stable isotope analysis is traditionally performed by bulk mass spectrometry. Although Raman spectroscopy distinguishes 12CO2 and 13CO2 isotopologue bands in spectra, using this technique to determine CO2 isotopic signature has been challenging. Here, we report on in-situ non-destructive analyses of the C stable isotopic composition of CO2, applying a novel high-resolution Raman configuration on 42 high-density CO2 fluid inclusions in mantle rocks from the Lake Tana region (Ethiopia) and El Hierro (Canary Islands). We collected two sets of three spectra with different acquisition times at high spectral resolution in each fluid inclusion. Among the 84 sets of spectra, 58 were characterised by integrated 13CO2/12CO2 band area ratios with reproducibility better than 4‰. Our results demonstrate the determination of δ13CCO2 by Raman spectroscopy in individual fluid inclusions with an error better than 2.5 ‰, which satisfactorily matches bulk mass spectrometry analyses in the same rock samples, supporting the accuracy of the measurements. We thus show that Raman Spectroscopy can provide a fundamental methodology for non-destructive, site-specific, and spatially resolved carbon isotope labelling at the microscale.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Deines, P. Early organic evolution. In Schidlowski M., Golubic S., Kimberley M. M., McKirdy D. M., Trudinger P. A. (eds) (Springer, Berlin, Heidelberg, 1992). 10.1007/978-3-642-76884-2_10.
-
- Deines P. The carbon isotope geochemistry of mantle xenoliths. Earth Sci. Rev. 2002;58:247–278. doi: 10.1016/S0012-8252(02)00064-8. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

