Standardized Extract of Valeriana officinalis Improves Overall Sleep Quality in Human Subjects with Sleep Complaints: A Randomized, Double-Blind, Placebo-Controlled, Clinical Study
- PMID: 37899385
- PMCID: PMC10796483
- DOI: 10.1007/s12325-023-02708-6
Standardized Extract of Valeriana officinalis Improves Overall Sleep Quality in Human Subjects with Sleep Complaints: A Randomized, Double-Blind, Placebo-Controlled, Clinical Study
Abstract
Introduction: Sleep deficit or poor sleep leads to ill-health, whereas sleep deprivation for longer periods of time increases the risk of developing adverse conditions associated with poor quality of life, and high socioeconomic impact. The treatments for sleep disturbances include melatonin and over-the-counter medicines like diphenhydramine and doxylamine, all of which have negative side effects. Valerian (Valeriana officinalis L.) is a traditional herb and the most preferred alternate sleep solution to manage sleep complaints.
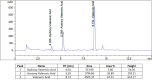
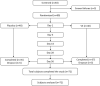
Methods: Eighty adult subjects with sleep complaints were randomized in 1:1 ratio to receive either V. officinalis extract (VE) or placebo for 8 weeks in a double-blind, placebo-controlled, parallel, clinical study. Primary efficacy endpoints included the Pittsburgh Sleep Quality Index (PSQI) and sleep latency using wrist actigraphy (WA), as well as a number of secondary endpoints, including sleep parameters such as actual sleep time and sleep efficiency using WA, the Epworth Sleepiness Scale (ESS), the Beck Anxiety Inventory (BAI), the Visual Analogue Scale (VAS) for the feeling of waking up refreshed, and a tertiary endpoint of sleep parameters using polysomnography (PSG) in a subset of 20 subjects per group. Safety parameters included physical examination, vital sign measurements, hematology, and clinical chemistry tests. Adverse events and serious adverse events were monitored throughout the study period.
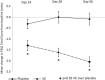
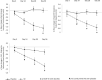
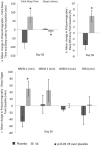
Results: Seventy-two subjects (35 and 37 subjects in the placebo and VE groups, respectively) completed the study and were included in the efficacy assessments. On Days 14, 28, and 56, the PSQI Total Score in the VE group decreased significantly (p < 0.05) compared to the placebo group. Further, the VE group showed significant improvements (p < 0.05) in sleep latency and actual sleep time on Days 3, 14, 28, and 56, and sleep efficiency on Days 14, 28, and 56, as evaluated by WA. There was a decrease (p < 0.05) in anxiety (BAI) on Days 14, 28, and 56, daytime drowsiness (ESS) on Days 28 and 56, and an increased feeling of waking up refreshed (VAS) on Days 28 and 56 compared to placebo. PSG results carried out in subset of subjects revealed significant improvements (p < 0.05) in total sleep time, sleep latency, and sleep efficiency on Day 56 in the VE group compared to the placebo group. No safety concerns were observed throughout the study.
Conclusion: VE supplementation significantly improved various subjective and objective parameters of sleep in young subjects with mild insomnia symptoms, such as overall sleep quality, sleep latency, sleep efficiency, and total sleep time. We also observed decreased anxiety and daytime sleepiness, and improved feeling of being refreshed after waking up with VE supplementation. VE was found to be safe and well tolerated throughout the study.
Trial registration: Clinical Trials Registry of India: CTRI/2022/05/042818.
Keywords: Anxiety; Insomnia; PSQI; Sleep quality; VAS; Valerian.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Valerian-hops combination and diphenhydramine for treating insomnia: a randomized placebo-controlled clinical trial.Sleep. 2005 Nov;28(11):1465-71. doi: 10.1093/sleep/28.11.1465. Sleep. 2005. PMID: 16335333 Clinical Trial.
-
Efficacy and safety of a polyherbal sedative-hypnotic formulation NSF-3 in primary insomnia in comparison to zolpidem: a randomized controlled trial.Indian J Pharmacol. 2013 Jan-Feb;45(1):34-9. doi: 10.4103/0253-7613.106432. Indian J Pharmacol. 2013. PMID: 23543804 Free PMC article. Clinical Trial.
-
Polysomnographic evaluation of the hypnotic effect of Valeriana edulis standardized extract in patients suffering from insomnia.Planta Med. 2001 Nov;67(8):695-9. doi: 10.1055/s-2001-18344. Planta Med. 2001. PMID: 11731907 Clinical Trial.
-
Treating primary insomnia - the efficacy of valerian and hops.Aust Fam Physician. 2010 Jun;39(6):433-7. Aust Fam Physician. 2010. PMID: 20628685 Review.
-
Valerian Root in Treating Sleep Problems and Associated Disorders-A Systematic Review and Meta-Analysis.J Evid Based Integr Med. 2020 Jan-Dec;25:2515690X20967323. doi: 10.1177/2515690X20967323. J Evid Based Integr Med. 2020. PMID: 33086877 Free PMC article.
Cited by
-
The effect of Valeriana officinalis tea on sympathovagal tone and cardiac function in healthy volunteers: A semi-experimental study.Avicenna J Phytomed. 2025 Jan-Feb;15(1):784-793. doi: 10.22038/AJP.2024.24974. Avicenna J Phytomed. 2025. PMID: 40271501 Free PMC article.
-
Integrative sleep management: from molecular pathways to conventional and herbal treatments.Naunyn Schmiedebergs Arch Pharmacol. 2025 May 8. doi: 10.1007/s00210-025-04183-y. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40338321 Review.
-
Impact of a Novel Valerian Extract on Sleep Quality, Relaxation, and GABA/Serotonin Receptor Activity in a Murine Model.Antioxidants (Basel). 2024 May 27;13(6):657. doi: 10.3390/antiox13060657. Antioxidants (Basel). 2024. PMID: 38929096 Free PMC article.
-
Efficacy and Tolerability of a Chemically Characterized Scutellaria lateriflora L. Extract-Based Food Supplement for Sleep Management: A Single-Center, Controlled, Randomized, Crossover, Double-Blind Clinical Trial.Nutrients. 2025 Apr 28;17(9):1491. doi: 10.3390/nu17091491. Nutrients. 2025. PMID: 40362800 Free PMC article. Clinical Trial.
-
Survey of perceived sleep quality and nursing implications in 172 adult ICU patients.Eur J Med Res. 2025 Aug 14;30(1):752. doi: 10.1186/s40001-025-03017-0. Eur J Med Res. 2025. PMID: 40814004 Free PMC article.
References
-
- Kumar VM. Sleep and sleep disorders. Indian J Chest Dis Allied Sci. 2008;50(1):129. - PubMed
-
- Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8):931–952. doi: 10.5664/jcsm.4950. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

