Impact of a reimbursement policy change on treatment with erenumab in migraine - a real-world experience from Germany
- PMID: 37899428
- PMCID: PMC10614330
- DOI: 10.1186/s10194-023-01682-2
Impact of a reimbursement policy change on treatment with erenumab in migraine - a real-world experience from Germany
Abstract
Background: Monoclonal antibodies (mAbs) targeting the Calcitonin Gene-Related Peptide (CGRP) pathway are safe and effective treatments for migraine prevention. However, the high cost of these novel therapies has led to reimbursement policies requiring patients to try multiple traditional preventives before access. In Germany, a recent change in insurance policy significantly expanded coverage for the CGRP receptor mAb erenumab, enabling migraine patients who failed just one prior prophylactic medication to receive this mAb. Here, we compare the clinical response to treatment with erenumab in migraine patients treated using the old and new coverage policy.
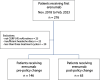
Methods: In this retrospective cohort study, we included CGRP-mAb naïve patients with episodic or chronic migraine, who started erenumab at our headache center according to either the old or the new insurance policy and received at least 3 consecutive injections. Headache diaries and electronic documentation were used to evaluate reductions in monthly headache and migraine days (MHD and MMD) and ≥ 50% and ≥ 30% responder rates at month 3 (weeks 9-12) of treatment.
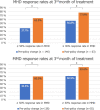
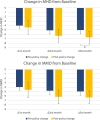
Results: We included 146 patients who received erenumab according to the old policy and 63 patients that were treated using the new policy. At weeks 9-12 of treatment, 37.7% of the old policy group had a 50% or greater reduction in MHD, compared to 63.5% of the new policy group (P < 0.001). Mean reduction in MHD was 5.02 days (SD = 5.46) and 6.67 days (SD = 5.32, P = 0.045) in the old and new policy cohort, respectively. After propensity score matching, the marginal effect of the new policy on treatment outcome was 2.29 days (standard error, SE: 0.715, P = 0.001) more reduction in MHD, and 30.1% (SE: 10.6%, P = 0.005) increase in ≥ 50% response rate for MHD.
Conclusions: Starting erenumab earlier in the course of migraine progression in a real-world setting may lead to a better response than starting after multiple failed prophylactic attempts. Continually gathering real-world evidence may help policymakers in deciding how readily to cover CGRP-targeted therapies in migraine prevention.
Keywords: Calcitonin gene-related peptide; Erenumab; Health policy; Insurance coverage; Migraine; Monoclonal antibodies; Preventive treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

