Cerebral Palsy - Early Diagnosis and Intervention Trial: protocol for the prospective multicentre CP-EDIT study with focus on diagnosis, prognostic factors, and intervention
- PMID: 37899466
- PMCID: PMC10614332
- DOI: 10.1186/s12887-023-04312-7
Cerebral Palsy - Early Diagnosis and Intervention Trial: protocol for the prospective multicentre CP-EDIT study with focus on diagnosis, prognostic factors, and intervention
Abstract
Background: Early diagnosis of cerebral palsy (CP) is important to enable intervention at a time when neuroplasticity is at its highest. Current mean age at diagnosis is 13 months in Denmark. Recent research has documented that an early-diagnosis set-up can lower diagnostic age in high-risk infants. The aim of the current study is to lower diagnostic age of CP regardless of neonatal risk factors. Additionally, we want to investigate if an early intervention program added to standard care is superior to standard care alone.
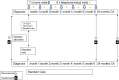
Methods: The current multicentre study CP-EDIT (Early Diagnosis and Intervention Trial) with the GO-PLAY intervention included (Goal Oriented ParentaL supported home ActivitY program), aims at testing the feasibility of an early diagnosis set-up and the GO-PLAY early intervention. CP-EDIT is a prospective cohort study, consecutively assessing approximately 500 infants at risk of CP. We will systematically collect data at inclusion (age 3-11 months) and follow a subset of participants (n = 300) with CP or at high risk of CP until the age of two years. The GO-PLAY early intervention will be tested in 80 infants with CP or high risk of CP. Focus is on eight areas related to implementation and perspectives of the families: early cerebral magnetic resonance imaging (MRI), early genetic testing, implementation of the General Movements Assessment method, analysis of the GO-PLAY early intervention, parental perspective of early intervention and early diagnosis, early prediction of CP, and comparative analysis of the Hand Assessment for Infants, Hammersmith Infant Neurological Examination, MRI, and the General Movements method.
Discussion: Early screening for CP is increasingly possible and an interim diagnosis of "high risk of CP" is recommended but not currently used in clinical care in Denmark. Additionally, there is a need to accelerate identification in mild or ambiguous cases to facilitate appropriate therapy early. Most studies on early diagnosis focus on identifying CP in infants below five months corrected age. Little is known about early diagnosis in the 50% of all CP cases that are discernible later in infancy. The current study aims at improving care of patients with CP even before they have an established diagnosis.
Trial registration: ClinicalTrials.gov ID 22013292 (reg. date 31/MAR/2023) for the CP-EDIT cohort and ID 22041835 (reg. date 31/MAR/2023) for the GO-PLAY trial.
Keywords: Brain imaging; Cerebral palsy; Early diagnosis; General movements assessment; Genomics; Hand assessment for infants; Intervention.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

