A Pilot Randomized Controlled Trial of De Novo Belatacept-based Immunosuppression After Lung Transplantation
- PMID: 37899481
- PMCID: PMC10922335
- DOI: 10.1097/TP.0000000000004841
A Pilot Randomized Controlled Trial of De Novo Belatacept-based Immunosuppression After Lung Transplantation
Abstract
Background: Chronic lung allograft dysfunction (CLAD) is the leading cause of death beyond the first year after lung transplantation. The development of donor-specific antibodies (DSA) is a recognized risk factor for CLAD. Based on experience in kidney transplantation, we hypothesized that belatacept, a selective T-cell costimulatory blocker, would reduce the incidence of DSA after lung transplantation, which may ameliorate the risk of CLAD.
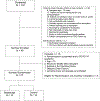
Methods: We conducted a pilot randomized controlled trial (RCT) at 2 sites to assess the feasibility and inform the design of a large-scale RCT. All participants were treated with rabbit antithymocyte globulin for induction immunosuppression. Participants in the control arm were treated with tacrolimus, mycophenolate mofetil, and prednisone, and participants in the belatacept arm were treated with tacrolimus, belatacept, and prednisone through day 89 after transplant then converted to belatacept, mycophenolate mofetil, and prednisone for the remainder of year 1.
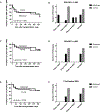
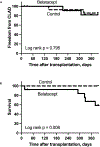
Results: After randomizing 27 participants, 3 in the belatacept arm died compared with none in the control arm. As a result, we stopped enrollment and treatment with belatacept, and all participants were treated with standard-of-care immunosuppression. Overall, 6 participants in the belatacept arm died compared with none in the control arm (log rank P = 0.008). We did not observe any differences in the incidence of DSA, acute cellular rejection, antibody-mediated rejection, CLAD, or infections between the 2 groups.
Conclusions: We conclude that the investigational regimen used in this pilot RCT is associated with increased mortality after lung transplantation.
Trial registration: ClinicalTrials.gov NCT03388008.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
M.A. is on the scientific advisory board of Immunocoer and One Lambda. V.P. is a consultant for PrecisCa. D.K. is on the scientific advisory board of Sana Biotechnology. R.H. received grant funding from Bristol Myers Squibb. The other authors declare no conflicts of interest.
Figures

References
-
- Zhu MZL, Levvey BJ, McGriffin DC, et al. An intention-to-treat view of lung transplantation for interstitial lung disease: Successful strategies to minimize waiting list and posttransplant mortality. Transplantation. 2022;106:188–199. - PubMed
-
- Raguragavan A, Jayabalan D, Saxena A. Health-related quality of life outcomes following single or bilateral lung transplantation: A systematic review. Transplantation. 2023; 107: 838–848. - PubMed
-
- Yu H, Bian T, Yu Z, et al. Bilateral lung transplantation provides better long-term survival and pulmonary function than single lung transplantation: A systematic review and meta-analysis. Transplantation. 2019; 103: 2634–2644. - PubMed
-
- Chambers DC, Cherikh WA, Harhay MO, et al. The International Thoracic Organ Translpant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation report – 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019; 38: 1042–1055. - PMC - PubMed
-
- Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005; 5: 131–138. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

