Insights from the protein interaction Universe of the multifunctional "Goldilocks" kinase DYRK1A
- PMID: 37900285
- PMCID: PMC10600473
- DOI: 10.3389/fcell.2023.1277537
Insights from the protein interaction Universe of the multifunctional "Goldilocks" kinase DYRK1A
Abstract
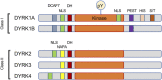
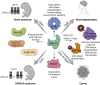
Human Dual specificity tyrosine (Y)-Regulated Kinase 1A (DYRK1A) is encoded by a dosage-dependent gene located in the Down syndrome critical region of human chromosome 21. The known substrates of DYRK1A include proteins involved in transcription, cell cycle control, DNA repair and other processes. However, the function and regulation of this kinase is not fully understood, and the current knowledge does not fully explain the dosage-dependent function of this kinase. Several recent proteomic studies identified DYRK1A interacting proteins in several human cell lines. Interestingly, several of known protein substrates of DYRK1A were undetectable in these studies, likely due to a transient nature of the kinase-substrate interaction. It is possible that the stronger-binding DYRK1A interacting proteins, many of which are poorly characterized, are involved in regulatory functions by recruiting DYRK1A to the specific subcellular compartments or distinct signaling pathways. Better understanding of these DYRK1A-interacting proteins could help to decode the cellular processes regulated by this important protein kinase during embryonic development and in the adult organism. Here, we review the current knowledge of the biochemical and functional characterization of the DYRK1A protein-protein interaction network and discuss its involvement in human disease.
Keywords: DCAF7; FAM117B; FAM53C; GLCCI1; RNF169; TROAP; development; proteomic analysis.
Copyright © 2023 Ananthapadmanabhan, Shows, Dickinson and Litovchick.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

