Clinical Guidelines for Diagnosis and Management of Cowden Syndrome/PTEN Hamartoma Tumor Syndrome in Children and Adults-Secondary Publication
- PMID: 37900693
- PMCID: PMC10600266
- DOI: 10.23922/jarc.2023-028
Clinical Guidelines for Diagnosis and Management of Cowden Syndrome/PTEN Hamartoma Tumor Syndrome in Children and Adults-Secondary Publication
Abstract
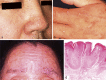
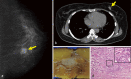
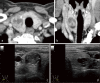
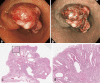
Cowden syndrome (CS)/PTEN hamartoma tumor syndrome (PHTS) is a rare autosomal dominantly inherited condition caused by germline pathogenesis. It is associated with multiple hamartomatous lesions occurring in various organs and tissues, including the gastrointestinal tract, skin, mucous membranes, breast, thyroid, endometrium, and brain. Macrocephaly or multiple characteristic mucocutaneous lesions commonly develop in individuals in their 20s. This syndrome is occasionally diagnosed in childhood due to the occurrence of multiple gastrointestinal polyps, autism spectrum disorders, and intellectual disability. CS/PHTS can be diagnosed taking the opportunity of multigene panel testing in patients with cancer. Appropriate surveillance for early diagnosis of associated cancers is required because patients have a high risk of cancers including breast, thyroid, colorectal, endometrial, and renal cancers. Under these circumstances, there is growing concern regarding the management of CS/PHTS in Japan, but there are no available practice guidelines. To address this situation, the guideline committee, which included specialists from multiple academic societies, was organized by the Research Group on Rare and Intractable Diseases granted by the Ministry of Health, Labour, and Welfare, Japan. The present clinical guidelines explain the principles in the diagnosis and management of CS/PHTS, together with four clinical questions and the corresponding recommendations, incorporating the concept of the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we present an English version of the guideline, some of which have been updated, to promote seamless implementation of accurate diagnosis and appropriate management of pediatric, adolescent, and adult patients with CS/PHTS.
Keywords: Cowden syndrome; PTEN hamartoma tumor syndrome; cancer; colorectal polyp.
Copyright © 2023 The Japan Society of Coloproctology.
Conflict of interest statement
Conflicts of Interest Prior to the preparation of these clinical guidelines, all members of the guidelines committee declared any conflicts of interest. TT received grants from Fujifilm Co. KB received payment for lectures from ASKA Pharmaceutical Holdings Co., Ltd. and grants from Sanofi, SA; Sysmex Co.; Taiho Pharmaceutical Co., Ltd.; KISSEI Pharma, Co., Ltd.; Fuji Pharma, Co., Ltd.; Astellas Pharma, Inc.; and HEARZEST Co., Ltd. YF has institutional COI of receiving grants from Onco Therapy Science, Inc. and the Uehara memorial foundation. NT received grants from Taiho Pharmaceutical Co. Ltd. HI received grants from Taiho Pharmaceutical Co. Ltd. and Yakult Honsha Co. Ltd. The other authors declare no conflicts of interest.
Figures



References
-
- Takayama T, Igarashi M, Ohsumi S, et al. Japanese Clinical Guidelines 2020 for Diagnosis and Treatment of Cowden Syndrome/PTEN Hamartoma Syndrome in Children and Adults. Journal of Hereditary Tumors. 2020 Sep; 20: 93-114. Japanese.
-
- Kojimahara N, Nakayama T, Morizane T, et al (Eds.). Minds manual for guideline development 2007. Tokyo: Japan Council for Quality Health Care; 2017. Japanese.
-
- Aihara M: GRADE system for medical guidelines -2nd edition. Hirosaki: Toppan Media; 2015. Japanese.
-
- Jaeschke R, Guyatt GH, Dellinger P, et al; GRADE Working Group. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008 Jul; 337: a744. - PubMed
-
- Nelen MR, Kremer H, Konings IB, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet. 1999 Apr; 7(3): 267-73. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

