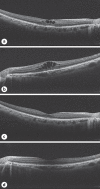
Cystoid Macular Edema following Treatment with Nanoparticle Albumin-Bound Paclitaxel and Atezolizumab for Metastatic Breast Cancer
- PMID: 37900858
- PMCID: PMC10601834
- DOI: 10.1159/000533999
Cystoid Macular Edema following Treatment with Nanoparticle Albumin-Bound Paclitaxel and Atezolizumab for Metastatic Breast Cancer
Abstract
Cystoid macular edema (CME) is a rare side effect associated with chemotherapy. Although the development of CME has been reported to occur following treatment with taxane drugs, such as nanoparticle albumin-bound paclitaxel (Nab-PTX), the occurrence of CME with treatment with atezolizumab has not yet been reported. Here, we report the case of a 49-year-old woman who developed CME 19 months into chemotherapy with Nab-PTX and atezolizumab. Improvement was not achieved with steroid injections into the Tenon's sac, and Nab-PTX and atezolizumab treatments were ceased. One month later, there was subjective improvement in her symptoms. Although many reports have indicated that cessation of chemotherapy has successfully improved CME, a specific treatment for CME has not yet been established. Clinicians should be aware of the ophthalmologic side effects and offer immediate treatment if symptoms develop.
Keywords: Atezolizumab; Breast cancer; Cystoid macular edema; Nanoparticle albumin-bound paclitaxel.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Intravitreal Ranibizumab Had Limited Effect on Cystoid Macular Edema Due to Albumin-Bound Paclitaxel: A Case Report and Literature Review.Front Oncol. 2021 Dec 13;11:773540. doi: 10.3389/fonc.2021.773540. eCollection 2021. Front Oncol. 2021. PMID: 34966680 Free PMC article.
-
Regression of taxane-related cystoid macular edema after topical dorzolamide treatment: two case reports.J Med Case Rep. 2021 Jul 21;15(1):355. doi: 10.1186/s13256-021-02954-8. J Med Case Rep. 2021. PMID: 34284818 Free PMC article.
-
[A Case of Cystoid Macular Edema Secondary to Albumin-Bound Paclitaxel Therapy].Gan To Kagaku Ryoho. 2017 Jul;44(7):599-602. Gan To Kagaku Ryoho. 2017. PMID: 28790265 Review. Japanese.
-
[Cystoid macular edema secondary to albumin-bound paclitaxel therapy for pancreatic cancer].Nihon Shokakibyo Gakkai Zasshi. 2021;118(3):272-278. doi: 10.11405/nisshoshi.118.272. Nihon Shokakibyo Gakkai Zasshi. 2021. PMID: 33692262 Japanese.
-
Nab-Paclitaxel related cystoid macular edema.Clin Ter. 2022 Jul-Aug;173(4):377-383. doi: 10.7417/CT.2022.2449. Clin Ter. 2022. PMID: 35857057 Review.
Cited by
-
Unraveling the Mystery of Taxol-Induced Cystoid Macular Oedema: Case Report and Literature Review.Rom J Ophthalmol. 2025 Jan-Mar;69(1):3-9. doi: 10.22336/rjo.2025.02. Rom J Ophthalmol. 2025. PMID: 40330966 Free PMC article. Review.
-
Adverse event profile of albumin-bound paclitaxel: a real-world pharmacovigilance analysis.Front Pharmacol. 2024 Oct 28;15:1448144. doi: 10.3389/fphar.2024.1448144. eCollection 2024. Front Pharmacol. 2024. PMID: 39529884 Free PMC article.
-
Efficacy of topical carbonic anhydrase inhibitors in treating taxane drug-induced cystoid macular edema: A case report.Medicine (Baltimore). 2025 Jan 3;104(1):e40958. doi: 10.1097/MD.0000000000040958. Medicine (Baltimore). 2025. PMID: 40184125 Free PMC article.
-
Bilateral macular edema secondary to nab-paclitaxel therapy for breast cancer.Int J Ophthalmol. 2024 Oct 18;17(10):1963-1966. doi: 10.18240/ijo.2024.10.26. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39430033 Free PMC article. No abstract available.
References
-
- Rizzo A, Ricci AD, Lanotte L, Lombardi L, Di Federico A, Brandi G, et al. . Immune-based combinations for metastatic triple negative breast cancer in clinical trials: current knowledge and therapeutic prospects. Expert Opin Investig Drugs. 2022;31(6):557–65. 10.1080/13543784.2022.2009456. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources