Unlocking the potential of Tregs: innovations in CAR technology
- PMID: 37900916
- PMCID: PMC10602912
- DOI: 10.3389/fmolb.2023.1267762
Unlocking the potential of Tregs: innovations in CAR technology
Abstract
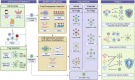
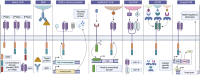
Regulatory T cells (Tregs) adoptive immunotherapy is emerging as a viable treatment option for both autoimmune and alloimmune diseases. However, numerous challenges remain, including limitations related to cell number, availability of target-specific cells, stability, purity, homing ability, and safety concerns. To address these challenges, cell engineering strategies have emerged as promising solutions. Indeed, it has become feasible to increase Treg numbers or enhance their stability through Foxp3 overexpression, post-translational modifications, or demethylation of the Treg-specific demethylated region (TSDR). Specificity can be engineered by the addition of chimeric antigen receptors (CARs), with new techniques designed to fine-tune specificity (tandem chimeric antigen receptors, universal chimeric antigen receptors, synNotch chimeric antigen receptors). The introduction of B-cell targeting antibody receptor (BAR) Tregs has paved the way for effective regulation of B cells and plasma cells. In addition, other constructs have emerged to enhance Tregs activation and function, such as optimized chimeric antigen receptors constructs and the use of armour proteins. Chimeric antigen receptor expression can also be better regulated to limit tonic signaling. Furthermore, various opportunities exist for enhancing the homing capabilities of CAR-Tregs to improve therapy outcomes. Many of these genetic modifications have already been explored for conventional CAR-T therapy but need to be further considered for CAR-Tregs therapies. This review highlights innovative CAR-engineering strategies that have the potential to precisely and efficiently manage immune responses in autoimmune diseases and improve transplant outcomes. As these strategies are further explored and optimized, CAR-Treg therapies may emerge as powerful tools for immune intervention.
Keywords: cell therapy; chimeric antigen receptor (CAR); genetic engineering; immunotherapy; regulatory T cells; tolerance.
Copyright © 2023 Requejo Cier, Valentini and Lamarche.
Conflict of interest statement
CL is an inventor on pending patents related to HLA-A2-specific CARs. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

