The G protein biased serotonin 5-HT2A receptor agonist lisuride exerts anti-depressant drug-like activities in mice
- PMID: 37900918
- PMCID: PMC10603247
- DOI: 10.3389/fmolb.2023.1233743
The G protein biased serotonin 5-HT2A receptor agonist lisuride exerts anti-depressant drug-like activities in mice
Abstract
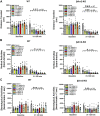
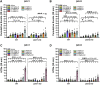
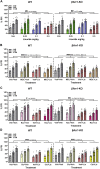
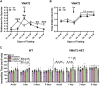
There is now evidence from multiple Phase II clinical trials that psychedelic drugs can exert long-lasting anxiolytic, anti-depressant, and anti-drug abuse (nicotine and ethanol) effects in patients. Despite these benefits, the hallucinogenic actions of these drugs at the serotonin 2A receptor (5-HT2AR) limit their clinical use in diverse settings. Activation of the 5-HT2AR can stimulate both G protein and β-arrestin (βArr) -mediated signaling. Lisuride is a G protein biased agonist at the 5-HT2AR and, unlike the structurally-related lysergic acid diethylamide (LSD), the drug does not typically produce hallucinations in normal subjects at routine doses. Here, we examined behavioral responses to lisuride, in wild-type (WT), βArr1-knockout (KO), and βArr2-KO mice. In the open field, lisuride reduced locomotor and rearing activities, but produced a U-shaped function for stereotypies in both βArr lines of mice. Locomotion was decreased overall in βArr1-KOs and βArr2-KOs relative to wild-type controls. Incidences of head twitches and retrograde walking to lisuride were low in all genotypes. Grooming was decreased in βArr1 mice, but was increased then decreased in βArr2 animals with lisuride. Serotonin syndrome-associated responses were present at all lisuride doses in WTs, but they were reduced especially in βArr2-KO mice. Prepulse inhibition (PPI) was unaffected in βArr2 mice, whereas 0.5 mg/kg lisuride disrupted PPI in βArr1 animals. The 5-HT2AR antagonist MDL100907 failed to restore PPI in βArr1 mice, whereas the dopamine D2/D3 antagonist raclopride normalized PPI in WTs but not in βArr1-KOs. Clozapine, SCH23390, and GR127935 restored PPI in both βArr1 genotypes. Using vesicular monoamine transporter 2 mice, lisuride reduced immobility times in tail suspension and promoted a preference for sucrose that lasted up to 2 days. Together, it appears βArr1 and βArr2 play minor roles in lisuride's actions on many behaviors, while this drug exerts anti-depressant drug-like responses without hallucinogenic-like activities.
Keywords: head twitch; lisuride; mice; prepulse inhibition; serotonin 2A receptor; serotonin-syndrome; β-arrestin.
Copyright © 2023 Pogorelov, Rodriguiz, Roth and Wetsel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
The G protein biased serotonin 5-HT 2A receptor agonist lisuride exerts anti-depressant drug-like activities in mice.bioRxiv [Preprint]. 2023 Jun 5:2023.06.01.543310. doi: 10.1101/2023.06.01.543310. bioRxiv. 2023. Update in: Front Mol Biosci. 2023 Oct 10;10:1233743. doi: 10.3389/fmolb.2023.1233743. PMID: 37333376 Free PMC article. Updated. Preprint.
Similar articles
-
Comparative Pharmacological Effects of Lisuride and Lysergic Acid Diethylamide Revisited.ACS Pharmacol Transl Sci. 2024 Feb 5;7(3):641-653. doi: 10.1021/acsptsci.3c00192. eCollection 2024 Mar 8. ACS Pharmacol Transl Sci. 2024. PMID: 38481684 Free PMC article.
-
The G protein biased serotonin 5-HT 2A receptor agonist lisuride exerts anti-depressant drug-like activities in mice.bioRxiv [Preprint]. 2023 Jun 5:2023.06.01.543310. doi: 10.1101/2023.06.01.543310. bioRxiv. 2023. Update in: Front Mol Biosci. 2023 Oct 10;10:1233743. doi: 10.3389/fmolb.2023.1233743. PMID: 37333376 Free PMC article. Updated. Preprint.
-
LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1.Sci Rep. 2021 Sep 3;11(1):17690. doi: 10.1038/s41598-021-96736-3. Sci Rep. 2021. PMID: 34480046 Free PMC article.
-
The neuropharmacology of sleep paralysis hallucinations: serotonin 2A activation and a novel therapeutic drug.Psychopharmacology (Berl). 2018 Nov;235(11):3083-3091. doi: 10.1007/s00213-018-5042-1. Epub 2018 Oct 5. Psychopharmacology (Berl). 2018. PMID: 30288594 Free PMC article. Review.
-
The physiological relevance of functional selectivity in dopamine signalling.Int J Obes Suppl. 2014 Jul;4(Suppl 1):S5-8. doi: 10.1038/ijosup.2014.3. Epub 2014 Jul 8. Int J Obes Suppl. 2014. PMID: 27152166 Free PMC article. Review.
Cited by
-
Comparative Pharmacological Effects of Lisuride and Lysergic Acid Diethylamide Revisited.ACS Pharmacol Transl Sci. 2024 Feb 5;7(3):641-653. doi: 10.1021/acsptsci.3c00192. eCollection 2024 Mar 8. ACS Pharmacol Transl Sci. 2024. PMID: 38481684 Free PMC article.
-
Psilocybin as Transformative Fast-Acting Antidepressant: Pharmacological Properties and Molecular Mechanisms.Fundam Clin Pharmacol. 2025 Aug;39(4):e70038. doi: 10.1111/fcp.70038. Fundam Clin Pharmacol. 2025. PMID: 40670864 Free PMC article. Review.
-
Antidepressant-like effects of psychedelics in a chronic despair mouse model: is the 5-HT2A receptor the unique player?Neuropsychopharmacology. 2024 Mar;49(4):747-756. doi: 10.1038/s41386-024-01794-6. Epub 2024 Jan 11. Neuropsychopharmacology. 2024. PMID: 38212441 Free PMC article.
-
Biological studies of clavine alkaloids targeting CNS receptors.Front Psychiatry. 2023 Nov 21;14:1286941. doi: 10.3389/fpsyt.2023.1286941. eCollection 2023. Front Psychiatry. 2023. PMID: 38076698 Free PMC article.
References
-
- Allen J. A., Yost J. M., Setola V., Chen X., Sassano M. F., Chen M., et al. (2011). Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. (USA) 108, 18488–18493. 10.1073/pnas.1104807108 - DOI - PMC - PubMed
-
- Bhatnagar A., Willins D. L., Gray J. A., Woods J., Benovic J. L., Roth B. L. (2001). The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J. Biol. Chem. 276, 8269–8277. 10.1074/jbc.M006968200 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

