Interferon-γ priming enhances the therapeutic effects of menstrual blood-derived stromal cells in a mouse liver ischemia-reperfusion model
- PMID: 37900937
- PMCID: PMC10600742
- DOI: 10.4252/wjsc.v15.i9.876
Interferon-γ priming enhances the therapeutic effects of menstrual blood-derived stromal cells in a mouse liver ischemia-reperfusion model
Abstract
Background: Mesenchymal stem cells (MSCs) have been used in liver transplantation and have certain effects in alleviating liver ischemia-reperfusion injury (IRI) and regulating immune rejection. However, some studies have indicated that the effects of MSCs are not very significant. Therefore, approaches that enable MSCs to exert significant and stable therapeutic effects are worth further study.
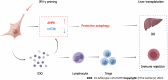
Aim: To enhance the therapeutic potential of human menstrual blood-derived stromal cells (MenSCs) in the mouse liver ischemia-reperfusion (I/R) model via interferon-γ (IFN-γ) priming.
Methods: Apoptosis was analyzed by flow cytometry to evaluate the safety of IFN-γ priming, and indoleamine 2,3-dioxygenase (IDO) levels were measured by quantitative real-time reverse transcription polymerase chain reaction, western blotting, and ELISA to evaluate the efficacy of IFN-γ priming. In vivo, the liver I/R model was established in male C57/BL mice, hematoxylin and eosin and TUNEL staining was performed and serum liver enzyme levels were measured to assess the degree of liver injury, and regulatory T cell (Treg) numbers in spleens were determined by flow cytometry to assess immune tolerance potential. Metabolomics analysis was conducted to elucidate the potential mechanism underlying the regulatory effects of primed MenSCs. In vitro, we established a hypoxia/reoxygenation (H/R) model and analyzed apoptosis by flow cytometry to investigate the mechanism through which primed MenSCs inhibit apoptosis. Transmission electron microscopy, western blotting, and immunofluorescence were used to analyze autophagy levels.
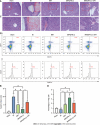
Results: IFN-γ-primed MenSCs secreted higher levels of IDO, attenuated liver injury, and increased Treg numbers in the mouse spleens to greater degrees than untreated MenSCs. Metabolomics and autophagy analyses proved that primed MenSCs more strongly induced autophagy in the mouse livers. In the H/R model, autophagy inhibitors increased the level of H/R-induced apoptosis, indicating that autophagy exerted protective effects. In addition, primed MenSCs decreased the level of H/R-induced apoptosis via IDO and autophagy. Further rescue experiments proved that IDO enhanced the protective autophagy by inhibiting the mammalian target of rapamycin (mTOR) pathway and activating the AMPK pathway.
Conclusion: IFN-γ-primed MenSCs exerted better therapeutic effects in the liver I/R model by secreting higher IDO levels. MenSCs and IDO activated the AMPK-mTOR-autophagy axis to reduce IRI, and IDO increased Treg numbers in the spleen and enhanced the MenSC-mediated induction of immune tolerance. Our study suggests that IFN-γ-primed MenSCs may be a novel and superior MSC product for liver transplantation in the future.
Keywords: Autophagy; Cell therapy; Liver; Mesenchymal stem cells; Reperfusion injury; T-lymphocytes.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures







Similar articles
-
Endometrial mesenchymal stem/stromal cells: The Enigma to code messages for generation of functionally active regulatory T cells.Stem Cell Res Ther. 2021 Oct 9;12(1):536. doi: 10.1186/s13287-021-02603-3. Stem Cell Res Ther. 2021. PMID: 34627370 Free PMC article.
-
Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis.J Tissue Eng Regen Med. 2019 Oct;13(10):1792-1804. doi: 10.1002/term.2930. Epub 2019 Jul 25. J Tissue Eng Regen Med. 2019. PMID: 31293088
-
Exosome from indoleamine 2,3-dioxygenase-overexpressing bone marrow mesenchymal stem cells accelerates repair process of ischemia/reperfusion-induced acute kidney injury by regulating macrophages polarization.Stem Cell Res Ther. 2022 Jul 28;13(1):367. doi: 10.1186/s13287-022-03075-9. Stem Cell Res Ther. 2022. PMID: 35902956 Free PMC article.
-
Therapeutic Efficacy of Interferon-Gamma and Hypoxia-Primed Mesenchymal Stromal Cells and Their Extracellular Vesicles: Underlying Mechanisms and Potentials in Clinical Translation.Biomedicines. 2024 Jun 20;12(6):1369. doi: 10.3390/biomedicines12061369. Biomedicines. 2024. PMID: 38927577 Free PMC article. Review.
-
Understanding menstrual blood-derived stromal/stem cells: Definition and properties. Are we rushing into their therapeutic applications?iScience. 2021 Nov 22;24(12):103501. doi: 10.1016/j.isci.2021.103501. eCollection 2021 Dec 17. iScience. 2021. PMID: 34917895 Free PMC article. Review.
Cited by
-
Enhancing the functionality of mesenchymal stem cells: An attractive treatment strategy for metabolic dysfunction-associated steatotic liver disease?World J Stem Cells. 2024 Oct 26;16(10):854-859. doi: 10.4252/wjsc.v16.i10.854. World J Stem Cells. 2024. PMID: 39493827 Free PMC article.
-
The interrelationship between interleukin-17 A and the immunomodulation of mesenchymal stromal cells.Stem Cell Res Ther. 2025 Sep 1;16(1):480. doi: 10.1186/s13287-025-04595-w. Stem Cell Res Ther. 2025. PMID: 40890871 Free PMC article. Review.
-
The Therapeutic Use and Potential of MSCs: Advances in Regenerative Medicine.Int J Mol Sci. 2025 Mar 27;26(7):3084. doi: 10.3390/ijms26073084. Int J Mol Sci. 2025. PMID: 40243782 Free PMC article. Review.
-
Priming mesenchymal stem cells to develop "super stem cells".World J Stem Cells. 2024 Jun 26;16(6):623-640. doi: 10.4252/wjsc.v16.i6.623. World J Stem Cells. 2024. PMID: 38948094 Free PMC article.
References
-
- Yang B, Duan W, Wei L, Zhao Y, Han Z, Wang J, Wang M, Dai C, Zhang B, Chen D, Chen Z. Bone Marrow Mesenchymal Stem Cell-Derived Hepatocyte-Like Cell Exosomes Reduce Hepatic Ischemia/Reperfusion Injury by Enhancing Autophagy. Stem Cells Dev. 2020;29:372–379. - PubMed
-
- Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y, Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33:1695–1710. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

