Achievement of Complete Response and Drug-Free Status by Atezolizumab plus Bevacizumab Combined with or without Curative Conversion in Patients with Transarterial Chemoembolization-Unsuitable, Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Proof-Of-Concept Study
- PMID: 37901197
- PMCID: PMC10603621
- DOI: 10.1159/000529574
Achievement of Complete Response and Drug-Free Status by Atezolizumab plus Bevacizumab Combined with or without Curative Conversion in Patients with Transarterial Chemoembolization-Unsuitable, Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Proof-Of-Concept Study
Abstract
Introduction: Atezolizumab plus bevacizumab therapy is extremely effective in the treatment of intermediate-stage hepatocellular carcinoma (HCC), with a response rate of 44%, as reported in the IMbrave150 trial. When tumor shrinkage is obtained, achieving complete response (CR) is possible in many cases using curative conversion with resection, ablation, or superselective transarterial chemoembolization (TACE) with curative intent. This concept, i.e., curative conversion by combining systemic therapy and locoregional therapy, has not been reported before. This multicenter proof-of-concept study was conducted to show the value of curative conversion in immunotherapy-treated intermediate-stage HCC meeting TACE-unsuitable criteria.
Methods: This study included 110 consecutive Child-Pugh A patients who received atezolizumab plus bevacizumab as first-line treatment for unresectable and TACE-unsuitable intermediate-stage HCC at seven centers in Japan. CR rate, drug-free rate, time to CR, change in liver function, efficacy in positron emission tomography (PET)-positive HCC, progression-free survival (PFS), and overall survival (OS) were assessed in patients who achieved CR using resection, ablation, superselective TACE with curative intent following atezolizumab plus bevacizumab or atezolizumab plus bevacizumab alone.
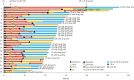
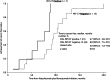
Results: Clinical or pathological CR was achieved in 38 patients (35%) (median observation period: 21.2 months). The modalities of curative conversion in 35 patients were as follows: resection, 7; ablation, 13; and superselective TACE, 15. Three patients achieved clinical CR with atezolizumab plus bevacizumab therapy alone. Among the 38 CR patients, 25 achieved drug-free status. PFS was not reached, and 3 patients experienced recurrence after reaching CR. Regarding OS, there were no deaths in any of the CR patients. The albumin-bilirubin score did not deteriorate after locoregional therapy or resection. Of seven PET-positive patients who achieved CR with atezolizumab plus bevacizumab followed by curative conversion, five achieved drug-free status.
Conclusion: The achievement of CR rate by curative conversion in patients treated with atezolizumab plus bevacizumab as the preceding therapy for unresectable and TACE-unsuitable intermediate-stage HCC was 35%. Overall, 23% of patients achieved drug-free status and no recurrence was observed from this patient subgroup with CR and drug-free status. Thus, achieving CR and/or drug-free status should be a therapeutic goal for patients with intermediate-stage HCC without vascular invasion or extrahepatic spread.
Keywords: Ablation; Atezolizumab plus bevacizumab; Cancer-free; Curative conversion; Hepatocellular carcinoma; Resection; Transarterial chemoembolization; Treatment-free.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Masatoshi Kudo received lecture fee from Eli Lilly, Bayer, Eisai, Chugai, Takeda, and MSD; and grants from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie, Eisai, Chugai, and GE Healthcare. Masatoshi Kudo is the editor-in-chief of Liver Cancer. Tomoko Aoki, Kazuomi Ueshima, Masahiro Morita, Hirokazu Chishina, Masahiro Takita, Satoru Hagiwara, Yasunori Minami, Hiroshi Ida, Naoshi Nishida (Smoking Research Foundation [Research Grant] Chikara Ogawa), Tetsu Tomonari, Noriaki Nakamura, Hidekatsu Kuroda, Atsushi Takebe, Yoshifumi Takeyama, Masaaki Hidaka, and Susumu Eguchi had no conflict of interest. Kaoru Tsuchiya received lecture fee from Eli Lilly, Bayer, Eisai, Chugai, and Takeda. Stephan L Chan is the advisor for Astra-Zeneca, MSD, Eisai, and Ipsen, and received research funding from Bayer, Eisai, Ipsen, Sirtex, and MSD. Masayuki Kurosaki received lecture fee from Gilead, AbbVie, Eli Lilly, Bayer, Eisai, Chugai, Janssen, and Otsuka. Namiki Izumi received lecturer fee from Chugai, Eisai, Takeda, Lily, and Bayer.
Figures





References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. 10.1016/S1470-2045(08)70285-7. - DOI - PubMed
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. 10.1016/S0140-6736(16)32453-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

