Targeting myeloid-derived suppressor cells with gemcitabine to enhance efficacy of adoptive cell therapy in bladder cancer
- PMID: 37901214
- PMCID: PMC10602731
- DOI: 10.3389/fimmu.2023.1275375
Targeting myeloid-derived suppressor cells with gemcitabine to enhance efficacy of adoptive cell therapy in bladder cancer
Abstract
Background: New therapeutics in development for bladder cancer need to address the recalcitrant nature of the disease. Intravesical adoptive cell therapy (ACT) with tumor infiltrating lymphocytes (TIL) can potentially induce durable responses in bladder cancer while maximizing T cells at the tumor site. T cells infused into the bladder directly encounter immunosuppressive populations, such as myeloid derived suppressor cells (MDSCs), that can attenuate T cell responses. Intravesical instillation of gemcitabine can be used as a lymphodepleting agent to precondition the bladder microenvironment for infused T cell products.
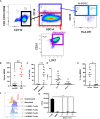
Methods: Urine samples from bladder cancer patients and healthy donors were analyzed by flow cytometry and cytometric bead array for immune profiling and cytokine quantification. MDSCs were isolated from the urine and cocultured with stimulated T cells to assess effects on proliferation. An orthotopic murine model of bladder cancer was established using the MB49-OVA cell line and immune profiling was performed. MDSCs from tumor-bearing mice were cocultured with OT-I splenocytes to assess T cell proliferation. Mice received intravesical instillation of gemcitabine and depletion of immune cells was measured via flow cytometry. Bladder tumor growth of mice treated with intravesical gemcitabine, OT-I transgenic T cells, or combination was monitored via ultrasound measurement.
Results: In comparison to healthy donors, urine specimen from bladder cancer patients show high levels of MDSCs and cytokines associated with myeloid chemotaxis, T cell chemotaxis, and inflammation. T cells isolated from healthy donors were less proliferative when cocultured with MDSCs from the urine. Orthotopic murine bladder tumors also presented with high levels of MDSCs along with enrichment of cytokines found in the patient urine samples. MDSCs isolated from spleens of tumor-bearing mice exerted suppressive effects on the proliferation of OT-I T cells. Intravesical instillation of gemcitabine reduced overall immune cells, MDSCs, and T cells in orthotopic bladder tumors. Combination treatment with gemcitabine and OT-I T cells resulted in sustained anti-tumor responses in comparison to monotherapy treatments.
Conclusion: MDSCs are enriched within the microenvironment of bladder tumors and are suppressive to T cells. Gemcitabine can be used to lymphodeplete bladder tumors and precondition the microenvironment for intravesical ACT.
Keywords: adoptive cell therapy (ACT); gemcitabine; lymphodepletion; neoadjuvant; non-muscle invasive bladder cancer (NMIBC); tumor infiltrating lymphocytes (TIL).
Copyright © 2023 Bazargan, Bunch, Ojwang‘, Blauvelt, Landin, Ali, Abrahams, Cox, Hall, Beatty, Poch, Rejniak and Pilon-Thomas.
Conflict of interest statement
BB is currently employed at Iovance Biotherapeutics. AH reports common stock holdings in AbbVie, Inc., Amgen, Inc., BioHaven Pharmaceuticals, and Bristol Myers Squibb. KR has received research support that is not related to this research from NIH-NCI R01CA272601 and R01CA230610. SP-T receives salary support on sponsored research agreements between Moffitt Cancer Center and Iovance Biotherapeutics, Turnstone Biologics, Intellia Therapeutics, and Dyve Biosciences. SP-T is a member of the SAB for KSQ Therapeutics, Inc. SP-T and MB are consultants for Morphogenesis, Inc. Moffitt Cancer Center and Research Institute has licensed intellectual property related to the proliferation and expansion of tumor infiltrating lymphocytes TIL to Iovance Biotherapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
-
- Sharma S, Ksheersagar P, Sharma P. Diagnosis and treatment of bladder cancer. Am Fam Physician (2009) 80(7):717–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

