Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection
- PMID: 37901217
- PMCID: PMC10600457
- DOI: 10.3389/fimmu.2023.1283120
Fc-mediated functions of nirsevimab complement direct respiratory syncytial virus neutralization but are not required for optimal prophylactic protection
Abstract
Introduction: Nirsevimab is an extended half-life (M252Y/S254T/T256E [YTE]-modified) monoclonal antibody to the pre-fusion conformation of the respiratory syncytial virus (RSV) Fusion protein, with established efficacy in preventing RSV-associated lower respiratory tract infection in infants for the duration of a typical RSV season. Previous studies suggest that nirsevimab confers protection via direct virus neutralization. Here we use preclinical models to explore whether fragment crystallizable (Fc)-mediated effector functions contribute to nirsevimab-mediated protection.
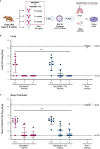
Methods: Nirsevimab, MEDI8897* (i.e., nirsevimab without the YTE modification), and MEDI8897*-TM (i.e., MEDI8897* without Fc effector functions) binding to Fc γ receptors (FcγRs) was evaluated using surface plasmon resonance. Antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent cellular phagocytosis (ADCP), antibody-dependent complement deposition (ADCD), and antibody-dependent cellular cytotoxicity (ADCC) were assessed through in vitro and ex vivo serological analyses. A cotton rat challenge study was performed with MEDI8897* and MEDI8897*-TM to explore whether Fc effector functions contribute to protection from RSV.
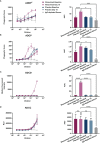
Results: Nirsevimab and MEDI8897* exhibited binding to a range of FcγRs, with expected reductions in FcγR binding affinities observed for MEDI8897*-TM. Nirsevimab exhibited in vitro ADNP, ADCP, ADCD, and ADCC activity above background levels, and similar ADNP, ADCP, and ADCD activity to palivizumab. Nirsevimab administration increased ex vivo ADNP, ADCP, and ADCD activity in participant serum from the MELODY study (NCT03979313). However, ADCC levels remained similar between nirsevimab and placebo. MEDI8897* and MEDI8897*-TM exhibited similar dose-dependent reduction in lung and nasal turbinate RSV titers in the cotton rat model.
Conclusion: Nirsevimab possesses Fc effector activity comparable with the current standard of care, palivizumab. However, despite possessing the capacity for Fc effector activity, data from RSV challenge experiments illustrate that nirsevimab-mediated protection is primarily dependent on direct virus neutralization.
Keywords: Fc-mediated effector function; RSV immunoprophylaxis; anti-RSV F protein monoclonal antibodies; nirsevimab; respiratory syncytial virus.
Copyright © 2023 Brady, Cayatte, Roe, Speer, Ji, Machiesky, Zhang, Wilkins, Tuffy and Kelly.
Conflict of interest statement
The authors of this manuscript are current or former employees of AstraZeneca and may hold AstraZeneca stock or stock options. The authors declare that this study received funding from AstraZeneca and Sanofi. The funders had the following involvement with the study: study design; collection, analysis and interpretation of data; the writing of this article and the decision to submit it for publication.
Figures

References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet (2022) 399(10340):2047–64. doi: 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet (2017) 390(10098):946–58. doi: 10.1016/S0140-6736(17)30938-8 - DOI - PMC - PubMed
-
- Arriola CS, Kim L, Langley G, Anderson EJ, Openo K, Martin AM, et al. Estimated burden of community-onset respiratory syncytial virus-associated hospitalizations among children aged <2 years in the United States, 2014-15. J Pediatr Infect Dis Soc (2020) 9(5):587–95. doi: 10.1093/jpids/piz087 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

