Updates in SJS/TEN: collaboration, innovation, and community
- PMID: 37901413
- PMCID: PMC10600400
- DOI: 10.3389/fmed.2023.1213889
Updates in SJS/TEN: collaboration, innovation, and community
Abstract
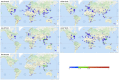
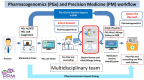
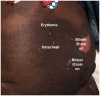
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN) is a predominantly drug-induced disease, with a mortality rate of 15-20%, that engages the expertise of multiple disciplines: dermatology, allergy, immunology, clinical pharmacology, burn surgery, ophthalmology, urogynecology, and psychiatry. SJS/TEN has an incidence of 1-5/million persons per year in the United States, with even higher rates globally. One of the challenges of SJS/TEN has been developing the research infrastructure and coordination to answer questions capable of transforming clinical care and leading to improved patient outcomes. SJS/TEN 2021, the third research meeting of its kind, was held as a virtual meeting on August 28-29, 2021. The meeting brought together 428 international scientists, in addition to a community of 140 SJS/TEN survivors and family members. The goal of the meeting was to brainstorm strategies to support the continued growth of an international SJS/TEN research network, bridging science and the community. The community workshop section of the meeting focused on eight primary themes: mental health, eye care, SJS/TEN in children, non-drug induced SJS/TEN, long-term health complications, new advances in mechanisms and basic science, managing long-term scarring, considerations for skin of color, and COVID-19 vaccines. The meeting featured several important updates and identified areas of unmet research and clinical need that will be highlighted in this white paper.
Keywords: HLA genotyping; SCORTEN; Stevens-Johnson Syndrome; Toxic Epidermal Necrolysis; body surface area; electronic medical record; pharmacogenomics; severe adverse cutaneous drug reactions.
Copyright © 2023 Marks, Botta, Abe, Beachkofsky, Boothman, Carleton, Chung, Cibotti, Dodiuk-Gad, Grimstein, Hasegawa, Hoofnagle, Hung, Kaffenberger, Kroshinsky, Lehloenya, Martin-Pozo, Micheletti, Mockenhaupt, Nagao, Pakala, Palubinsky, Pasieka, Peter, Pirmohamed, Reyes, Saeed, Shupp, Sukasem, Syu, Ueta, Zhou, Chang, Becker, Bellon, Bonnet, Cavalleri, Chodosh, Dewan, Dominguez, Dong, Ezhkova, Fuchs, Goldman, Himed, Mallal, Markova, McCawley, Norton, Ostrov, Phan, Sanford, Schlundt, Schneider, Shear, Shinkai, Tkaczyk, Trubiano, Volpi, Bouchard, Divito and Phillips.
Conflict of interest statement
KM was employed by Stevens-Johnson Syndrome Foundation. EJP reports grants from National Institutes of Health (R01HG010863, R01AI152183, U01AI154659) and from the National Health and Medical Research Council of Australia. She receives Royalties and consulting fees from UpToDate and has received consulting fees from Janssen, Verve, Biocryst, Regeneron, AstraZeneca and Novavax. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer OI declared a shared affiliation with the author AS to the handling editor at the time of review.
Figures


References
-
- Peter JG, Lehloenya R, Dlamini S, Risma K, White KD, Konvinse KC, et al. Severe delayed cutaneous and systemic reactions to drugs: a global perspective on the science and art of current practice. J Allergy Clin Immunol Pract. (2017) 5:547–63. doi: 10.1016/j.jaip.2017.01.025, PMID: - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

