Adaptive laboratory evolution of a thermophile toward a reduced growth temperature optimum
- PMID: 37901835
- PMCID: PMC10601643
- DOI: 10.3389/fmicb.2023.1265216
Adaptive laboratory evolution of a thermophile toward a reduced growth temperature optimum
Abstract
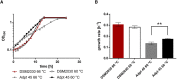
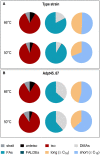
Thermophily is an ancient trait among microorganisms. The molecular principles to sustain high temperatures, however, are often described as adaptations, somewhat implying that they evolved from a non-thermophilic background and that thermophiles, i.e., organisms with growth temperature optima (TOPT) above 45°C, evolved from mesophilic organisms (TOPT 25-45°C). On the contrary, it has also been argued that LUCA, the last universal common ancestor of Bacteria and Archaea, may have been a thermophile, and mesophily is the derived trait. In this study, we took an experimental approach toward the evolution of a mesophile from a thermophile. We selected the acetogenic bacterium T. kivui (TOPT 66°C) since acetogenesis is considered ancient physiology and cultivated it at suboptimal low temperatures. We found that the lowest possible growth temperature (TMIN) under the chosen conditions was 39°C. The bacterium was subsequently subjected to adaptive laboratory evolution (ALE) by serial transfer at 45°C. Interestingly, after 67 transfers (approximately 180 generations), the adapted strain Adpt45_67 did not grow better at 45°C, but a shift in the TOPT to 60°C was observed. Growth at 45°C was accompanied by a change in the morphology as shorter, thicker cells were observed that partially occurred in chains. While the proportion of short-chain fatty acids increased at 50°C vs. 66°C in both strains, Adpt45_67 also showed a significantly increased proportion of plasmalogens. The genome analysis revealed 67 SNPs compared to the type strain, among these mutations in transcriptional regulators and in the cAMP binding protein. Ultimately, the molecular basis of the adaptation of T. kivui to a lower TOPT remains to be elucidated. The observed change in phenotype is the first experimental step toward the evolution of thermophiles growing at colder temperatures and toward a better understanding of the cold adaptation of thermophiles on early Earth.
Keywords: Thermoanaerobacter kivui; acetogens; adaptive laboratory evolution; cold adaptation; origin of life; thermophiles.
Copyright © 2023 Lehmann, Prohaska, Zeldes, Poehlein, Daniel and Basen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

