Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer's disease: A systematic review
- PMID: 37902139
- PMCID: PMC10617290
- DOI: 10.1177/03946320231209839
Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer's disease: A systematic review
Abstract
Background: Lecanemab is the latest monoclonal antibody that targets beta-amyloid approved exclusively for treatment of Alzheimer's disease with mild cognitive impairment or mild dementia. This article aims to provide a systematic review of the efficacy, and safety of lecanemab in slowing clinical decline in Alzheimer's disease.
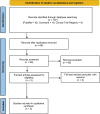
Methods: A comprehensive search of various databases, including the National Institute of Health clinical trials registry, PubMed, and the Cochrane library, was conducted until July 2023 using the keywords lecanemab, BAN2401, and Alzheimer's disease. Additionally, conference abstracts listed in the Cochrane database (including Embase) and drug information from the US Food and Drug Administration (FDA) label were examined. Only clinical trials published in the English language were considered. In total, 107 articles were retrieved, and after thorough evaluation, three randomized, double-blind, multicenter clinical trials involving 2729 participants were included in the analysis.
Results: The FDA approved lecanemab for Alzheimer's disease in January 2023 which acts as a novel disease-modifying anti-amyloid-beta (Aβ) human monoclonal antibody and is administered intravenously. Based on the clinical trials included in this review, lecanemab was found efficacious in reducing the accumulation of beta-amyloid and slowing down the cognitive decline and it was well tolerated. Lecanemab had a statistically significant change from baseline in Clinical Dementia Rating-Sum of Boxes (CDR-SB), Alzheimer's Disease Composite Score (ADCOMS), Alzheimer's Disease Assessment Scale (ADAScog14), Alzheimer's Disease Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL), and reductions in brain amyloid burden. The most common treatment-emergent adverse events were headache, infusion-related reactions, and Amyloid related imaging abnormalities-edema.
Conclusions: Lecanemab therapy led to a substantial decrease in amyloid plaques and a noticeable slowing of clinical decline. The findings suggest a meaningful connection between the reduction in amyloid and the positive impact on patients' clinical outcomes, hinting at potential disease-modifying effects.
Keywords: Alzheimer's disease; amyloid-beta antagonist; dementia; lecanemab.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Lord A, Gumucio A, Englund H, et al. An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer's disease. Neurobiology of disease 2009; 36(3):425–434. - PubMed
-
- U.S. Food and Drug Administration . FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. Available at: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerat...
-
- U.S. Food and Drug Administration. (2023) FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. Available at: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-a...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical