Concordance between patient-reported and physician-documented comorbidities and symptoms among Stage 4 breast cancer patients
- PMID: 37902219
- PMCID: PMC10709717
- DOI: 10.1002/cam4.6632
Concordance between patient-reported and physician-documented comorbidities and symptoms among Stage 4 breast cancer patients
Abstract
Background: Comorbidities and symptoms in metastatic breast cancer patients impact treatment decisions and influence prognosis and quality of life. The objective of this study is to examine the concordance between physician documentation and patient reports of comorbidities and symptoms to understand their comparative effectiveness.
Methods: New patients with metastatic breast cancer completed an electronic intake survey assessing patient health history and symptoms. Physician documentation across 54 comorbidities and 42 symptoms was abstracted from notes for the corresponding clinic visits between November 2016 and March 2020. Concordance between patient reports and medical records for each condition and hazards ratios for each patient versus physician reported comorbidity and symptom were assessed.
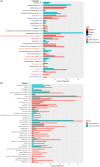
Results: A total of 168 patients were included in the analysis (age, median = 56 years, range = 29-86 years; 131 white [78.9%]). Twenty-three of 54 comorbidities had a moderate to high level of agreement between patients and physicians (κ ≥ 0.40). Physicians documented higher numbers of comorbidities that can be objectively measured which also had higher concordance (e.g., diabetes [κ = 0.83] and hypertension [κ = 0.79]) while patients reported higher numbers of comorbidities that are more subjective which also had lower concordance (anxiety [κ = 0.30], GERD [κ = 0.36]). One physician-documented and two patient-reported comorbidities were significantly associated with survival (p < 0.05). Only 2 of 42 symptoms had a moderate to high level of agreement between patients and physicians. One physician-documented and nine patient-reported symptoms were significantly associated with decreased survival (p < 0.05).
Conclusion: Agreement between patients' and physicians' reporting of comorbidities varies substantially, and patient reports can complement physician documentation. Physicians significantly underreported symptoms versus patients; thus, concordance was also low. Multiple patient-reported symptoms were predictive of survival; thus, incorporating them can provide more informative estimates of predicted survival.
Keywords: breast cancer; comorbidity; electronic health records; patient reported outcome measures; symptom assessment.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337‐350. - PubMed
-
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73‐84. - PubMed
-
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. - PubMed
-
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. - PubMed

