Solvent-Assisted Laser Desorption Flexible Microtube Plasma Mass Spectrometry for Direct Analysis of Dried Samples on Paper
- PMID: 37902451
- PMCID: PMC10733904
- DOI: 10.1021/acs.analchem.3c03009
Solvent-Assisted Laser Desorption Flexible Microtube Plasma Mass Spectrometry for Direct Analysis of Dried Samples on Paper
Abstract
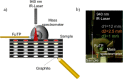
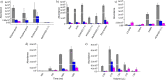
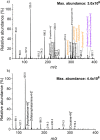
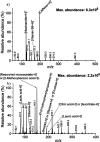
The present study investigated the potential for solvent-assisted laser desorption coupled with flexible microtube plasma ionization mass spectrometry (SALD-FμTP-MS) as a rapid analytical technique for direct analysis of surface-deposited samples. Paper was used as the demonstrative substrate, and an infrared hand-held laser was employed for sample desorption, aiming to explore cost-effective sampling and analysis methods. SALD-FμTP-MS offers several advantages, particularly for biofluid analysis, including affordability, the ability to analyze low sample volumes (<10 μL), expanded chemical coverage, sample and substrate stability, and in situ analysis and high throughput potential. The optimization process involved exploring the use of viscous solvents with high boiling points as liquid matrices. This approach aimed to enhance desorption and ionization efficiencies. Ethylene glycol (EG) was identified as a suitable solvent, which not only improved sensitivity but also ensured substrate stability during analysis. Furthermore, the addition of cosolvents such as acetonitrile/water (1:1) and ethyl acetate further enhanced sensitivity and reproducibility for a standard solution containing amphetamine, imazalil, and cholesterol. Optimized conditions for reproducible and sensitive analysis were determined as 1000 ms of laser exposure time using a 1 μL solvent mixture of 60% EG and 40% acetonitrile (ACN)/water (1:1). A mixture of 60% EG and 40% ACN/water (1:1) resulted in signal enhancements and relative standard deviations of 12, 20, and 13% for the evaluated standards, respectively. The applicability of SALD-FμTP-MS was further evaluated by successfully analyzing food, water, and biological samples, highlighting the potential of SALD-FμTP-MS analysis, particularly for thermolabile and polarity diverse compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Detection and Evaluation of Lipid Classes and Other Hydrophobic Compounds Using a Laser Desorption/Plasma Ionization Interface.Anal Chem. 2020 Nov 17;92(22):15212-15220. doi: 10.1021/acs.analchem.0c03839. Epub 2020 Nov 2. Anal Chem. 2020. PMID: 33135875
-
Evaluation of matrix-assisted laser desorption/ionization (MALDI) preparation techniques for surface characterization of intact Fusarium spores by MALDI linear time-of-flight mass spectrometry.Rapid Commun Mass Spectrom. 2009 Mar;23(6):877-84. doi: 10.1002/rcm.3949. Rapid Commun Mass Spectrom. 2009. PMID: 19224532
-
LC coupled to ESI, MALDI and ICP MS - A multiple hyphenation for metalloproteomic studies.Anal Chim Acta. 2017 May 22;968:58-65. doi: 10.1016/j.aca.2017.03.016. Epub 2017 Mar 24. Anal Chim Acta. 2017. PMID: 28395775
-
Surface analysis of lipids by mass spectrometry: more than just imaging.Prog Lipid Res. 2013 Oct;52(4):329-53. doi: 10.1016/j.plipres.2013.04.005. Epub 2013 Apr 24. Prog Lipid Res. 2013. PMID: 23623802 Review.
-
How to make big molecules fly out of liquid water: applications, features and physics of laser assisted liquid phase dispersion mass spectrometry.Phys Chem Chem Phys. 2007 Jul 14;9(26):3335-60. doi: 10.1039/b615114k. Epub 2007 Mar 27. Phys Chem Chem Phys. 2007. PMID: 17664960 Review.
Cited by
-
Direct sub-atmospheric pressure ionization mass spectrometry: Evaporation/sublimation-driven ionization is amazing, fundamentally, and practically.J Mass Spectrom. 2024 Jun;59(6):e5018. doi: 10.1002/jms.5018. J Mass Spectrom. 2024. PMID: 38736378 Free PMC article. Review.
References
-
- Pan Y.; Mao K.; Hui Q.; Wang B.; Cooper J.; Yang Z. Paper-based devices for rapid diagnosis and wastewater surveillance. TrAC, Trends Anal. Chem. 2022, 157, 11676010.1016/j.trac.2022.116760. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

