GTP Signaling Links Metabolism, DNA Repair, and Responses to Genotoxic Stress
- PMID: 37902550
- PMCID: PMC10872631
- DOI: 10.1158/2159-8290.CD-23-0437
GTP Signaling Links Metabolism, DNA Repair, and Responses to Genotoxic Stress
Abstract
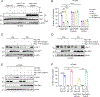
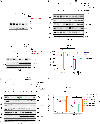
How cell metabolism regulates DNA repair is incompletely understood. Here, we define a GTP-mediated signaling cascade that links metabolism to DNA repair and has significant therapeutic implications. GTP, but not other nucleotides, regulates the activity of Rac1, a guanine nucleotide-binding protein, which promotes the dephosphorylation of serine 323 on Abl-interactor 1 (Abi-1) by protein phosphatase 5 (PP5). Dephosphorylated Abi-1, a protein previously not known to activate DNA repair, promotes nonhomologous end joining. In patients and mouse models of glioblastoma, Rac1 and dephosphorylated Abi-1 mediate DNA repair and resistance to standard-of-care genotoxic treatments. The GTP-Rac1-PP5-Abi-1 signaling axis is not limited to brain cancer, as GTP supplementation promotes DNA repair and Abi-1-S323 dephosphorylation in nonmalignant cells and protects mouse tissues from genotoxic insult. This unexpected ability of GTP to regulate DNA repair independently of deoxynucleotide pools has important implications for normal physiology and cancer treatment.
Significance: A newly described GTP-dependent signaling axis is an unexpected link between nucleotide metabolism and DNA repair. Disrupting this pathway can overcome cancer resistance to genotoxic therapy while augmenting it can mitigate genotoxic injury of normal tissues. This article is featured in Selected Articles from This Issue, p. 5.
©2023 American Association for Cancer Research.
Figures





Update of
-
GTP signaling links metabolism, DNA repair, and responses to genotoxic stress.bioRxiv [Preprint]. 2023 Apr 13:2023.04.12.536297. doi: 10.1101/2023.04.12.536297. bioRxiv. 2023. Update in: Cancer Discov. 2024 Jan 12;14(1):158-175. doi: 10.1158/2159-8290.CD-23-0437. PMID: 37090571 Free PMC article. Updated. Preprint.
References
-
- Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol 2010;12(2):116–21 doi 10.1093/neuonc/nop020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA148828/CA/NCI NIH HHS/United States
- R01 DK095201/DK/NIDDK NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- F32 CA260735/CA/NCI NIH HHS/United States
- R01 NS129123/NS/NINDS NIH HHS/United States
- R01 CA244931/CA/NCI NIH HHS/United States
- R35 HL144979/HL/NHLBI NIH HHS/United States
- R37 CA237421/CA/NCI NIH HHS/United States
- K08 CA234416/CA/NCI NIH HHS/United States
- R01 CA245546/CA/NCI NIH HHS/United States
- R37 CA258346/CA/NCI NIH HHS/United States
- L30 CA209251/CA/NCI NIH HHS/United States
- P50 CA269022/CA/NCI NIH HHS/United States
- R01 CA240515/CA/NCI NIH HHS/United States
- R01 CA248160/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

