Atrial Fibrillation In Patients With Stroke Attributed to Large- or Small-Vessel Disease: 3-Year Results From the STROKE AF Randomized Clinical Trial
- PMID: 37902733
- PMCID: PMC10616765
- DOI: 10.1001/jamaneurol.2023.3931
Atrial Fibrillation In Patients With Stroke Attributed to Large- or Small-Vessel Disease: 3-Year Results From the STROKE AF Randomized Clinical Trial
Abstract
Importance: The STROKE AF study found that in patients with prior ischemic stroke attributed to large-artery atherosclerotic disease (LAD) or small-vessel occlusive disease (SVD), 12% developed AF over 1 year when monitored with an insertable cardiac monitor (ICM). The occurrence over subsequent years is unknown.
Objectives: To compare the rates of AF detection through 3 years of follow-up between an ICM vs site-specific usual care in patients with prior ischemic stroke attributed to LAD or SVD.
Design, setting, and participants: This multicenter, randomized (1:1) clinical trial took place at 33 sites in the US with enrollment between April 2016 and July 2019 and 3-year follow-up through July 2022. Eligible patients were aged 60 years or older, or aged 50 to 59 years with at least 1 additional stroke risk factor and had an index ischemic stroke attributed to LAD or SVD within 10 days prior to ICM insertion. Of the 496 patients enrolled, 492 were randomized and 4 were excluded.
Interventions: ICM monitoring vs site-specific usual care.
Main outcomes and measures: The prespecified long-term outcome of the trial was AF detection through study follow-up (up to 3 years). AF was defined as an episode lasting more than 30 seconds, adjudicated by an expert committee.
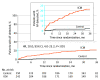
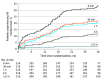
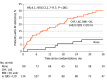
Results: In total, 492 patients were randomized and included in the analyses (median [IQR] age, 66 [60-74] years; 307 men [62.4%] and 185 women [37.6%]), of whom 314 completed 3-year follow-up (63.8%). The incidence rate of AF at 3 years was 21.7% (46 patients) in the ICM group vs 2.4% (5 patients) in the control group (hazard ratio, 10.0; 95% CI, 4.0-25.2; P < .001).
Conclusions and relevance: Patients with ischemic stroke attributed to LAD or SVD face an increasing risk of AF over time and most of the AF occurrences are not reliably detected by standard medical monitoring methods. One year of negative monitoring should not reassure clinicians that patients who have experienced stroke will not develop AF over the next 2 years.
Trial registration: ClinicalTrials.gov Identifier: NCT02700945.
Conflict of interest statement
Figures
Comment in
-
Looking for Atrial Fibrillation With Insertable Cardiac Monitors-Is 1 Year Long Enough and Does it Matter?JAMA Neurol. 2023 Dec 1;80(12):1266-1268. doi: 10.1001/jamaneurol.2023.3377. JAMA Neurol. 2023. PMID: 37902778 No abstract available.
References
-
- Bernstein RA, Kamel H, Granger CB, et al. ; STROKE-AF Investigators . Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE-AF randomized clinical trial. JAMA. 2021;325(21):2169-2177. doi: 10.1001/jama.2021.6470 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

