Targeted randomization dose optimization trials enable fractional dosing of scarce drugs
- PMID: 37903093
- PMCID: PMC10615276
- DOI: 10.1371/journal.pone.0287511
Targeted randomization dose optimization trials enable fractional dosing of scarce drugs
Abstract
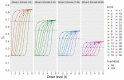
Administering drug at a dose lower than that used in pivotal clinical trials, known as fractional dosing, can stretch scarce resources. Implementing fractional dosing with confidence requires understanding a drug's dose-response relationship. Clinical trials aimed at describing dose-response in scarce, efficacious drugs risk underdosing, leading dose-finding trials to not be pursued despite their obvious potential benefit. We developed a new set of response-adaptive randomized dose-finding trials and demonstrate, in a series of simulated trials across diverse dose-response curves, these designs' efficiency in identifying the minimum dose that achieves satisfactory efficacy. Compared to conventional designs, these trials have higher probabilities of identifying lower doses while reducing the risks of both population- and subject-level underdosing. We strongly recommend that, upon demonstration of a drug's efficacy, pandemic drug development swiftly proceeds with response-adaptive dose-finding trials. This unified strategy ensures that scarce effective drugs produce maximum social benefits.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
PSB has received research funding from Bristol Myers Squibb and Janssen outside of the submitted work. AT has no conflicts to disclose. GWS serves as an uncompensated Director of the Optimal Cancer Care Alliance. GWS is a co-inventor of a patent held by the University of Chicago covering the use of low-dose tocilizumab in the treatment of viral infections. GWS reports no material conflicts of interest with regards to contract research organizations, biostatistical firms, or vaccines. GWS is employed by the United States Department of Veterans Affairs; this work does not represent the official position of the United States Department of Veterans Affairs. There are no patents, products in development or marketed products to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

